Siemens Healthineers and Mentice AB have announced that they are collaborating to fully integrate Mentice’s VIST virtual patient into the Artis icono angiography system from Siemens Healthineers.
Micro Medical Solutions has received Investigational Device Exemption (IDE) approval from the US Food and Drug Administration (FDA). IDE approval allows the company to initiate a US pivotal clinical trial to evaluate MicroStent’s safety and efficacy. A press release reports that MicroStentis a vascular stent specifically designed to achieve and maintain vessel patency and improve blood flow in order to reduce below-the-knee amputations for patients with critical limb ischaemia resulting from peripheral arterial disease.
The OVER trial investigators, led by the late Frank Lederle, recently released the long-term results of endovascular aneurysm repair (EVAR) versus open repair. Janet Powell writes for Vascular News about the 14-year outcomes, which were published in the New England Journal of Medicine, how they compare to other similar randomised controlled trials’ results, and what the data contribute to the ongoing conversation about the durability of EVAR.
According to a recent study, an endovascular approach is “safe and effective” in the treatment of abdominal aortic aneurysms detected by the National Abdominal Aortic Aneurysm Screening Programme (NAAASP). Furthermore, the investigators found no aneurysm-related mortality in those treated electively for infrarenal aneurysms, and the overall reintervention rate was “relatively low”.
The Cardioform ASD Occluder (Gore Medical) has received US Food and Drug Administration (FDA) premarket approval (PMA) for use in the percutaneous closure of ostium secundum atrial septal defects (ASDs). A press release from the company says that the FDA approval was supported by data collected from the pivotal stage of the Gore ASSURED clinical study which demonstrated 100% closure success at six-months in patients with a successful implant.
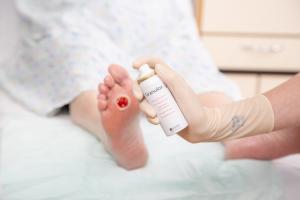
A new Expert Panel Report, published in The Diabetic Foot Journal, recommends a new treatment algorithm for topical oxygen therapy in patients with diabetic foot ulcers (DFU). The new treatment algorithm recommends that topical oxygen therapy is considered as an adjunct to standard of care after four weeks if the wound area has not reduced by 40%. In addition, it recommends that early initiation (<4 weeks) of topical oxygen therapy could be beneficial for patients where peripheral arterial disease (PAD) is present, where there is ulcer pain, non-healing after amputation, or sloughy wounds.
Vikram S Kashyap, co-principal investigator of the ROADSTER 2 trial, today presented the final 30-day outcomes of the post-market approval study in a late-breaking trial session at the Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM; 12–15 June, National Harbor, USA). The data show the sustained safety and efficacy of transcarotid artery revascularisation (TCAR) with Silk Road Medical’s Enroute system. In this interview, Kashyap speaks to Vascular News about the procedure, and how TCAR trialists are hoping to make an impact for patients needing carotid revascularisation.
The halted BASIL-3 study now plans to resume enrolment of patients into the randomised controlled trial, which is comparing balloon angioplasty versus stenting for severe ischaemia in the legs of patients with peripheral arterial disease (PAD). Two of the three trial arms use paclitaxel-coated or -eluting devices.
The Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI), with its large multicentre registries has produced several datasets for analysis of vascular patient care in the USA. This year, at the SVS Vascular Annual Meeting (VAM; 12–15 June, National Harbor, USA), new data produced with the use of the VQI was presented by Daniel Bertges (University of Vermont Medical Center, Burlington, USA) aimed to provide further insight into mortality following peripheral vascular interventions using paclitaxel devices. This study “highlights the potential use of the VQI for surveillance of the safety of new peripheral arterial devices”, the investigators state in their abstract.
The TLC-NOSF (UrgoStart, Urgo Medical) advanced wound care dressing was recently recommended by the UK National Institute for Health and Care Excellence (NICE) for healing of venous and diabetic foot ulcers. Speaking at the European Wound Management Association annual meeting (EWMA; 5–7 June, Gothenburg, Sweden)
The methodology underpinning the conclusion by Konstantinos Katsanos (Patras, Greece) et al that there is a positive dose-response relationship between paclitaxel and mortality is flawed, argues Andrew Holden (Auckland, New Zealand) and colleagues in a paper published in the Journal of Endovascular Therapy (JEVT). Holden and co-authors list the limitations they see in the summary level meta-analysis published in December 2018 in the Journal of the American Heart Association (JAHA), and call for physicians to “significantly modify the way vascular device trials are performed in the future”.
Thrombolex has announced that the US Food and Drug Administration (FDA) has cleared the Bashir Endovascular Catheter (BEC) for the controlled and selective infusion of physician-specified fluids, including thrombolytics, into the peripheral vasculature, and the Bashir N-X Endovascular Catheter (BEC N-X) for the controlled and selective infusion of physician-specified fluids into the peripheral and pulmonary artery vasculature.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389