Background
The incidence of digitalis toxicity has declined in recent years, due to decreased use of this drug along with improved technology for monitoring of drug levels and increased awareness of drug interactions. Nevertheless, cardiac glycoside toxicity continues to be a problem in the United States because of the wide use of digoxin (a preparation of digitalis) and its narrow therapeutic window.
The incidence of digitalis toxicity has declined in recent years, due to decreased use of this drug along with improved technology for monitoring of drug levels and increased awareness of drug interactions. Nevertheless, cardiac glycoside toxicity continues to be a problem in the United States because of the wide use of digoxin (a preparation of digitalis) and its narrow therapeutic window.
Digitalis is a plant-derived cardiac glycoside commonly used in the treatment of chronic heart failure (CHF), atrial fibrillation, and reentrant supraventricular tachycardia. Digoxin is the only available preparation of digitalis in the United States. (See Etiology and Epidemiology.)
Cardiac glycosides are found in certain flowering plants, such as oleander and lily-of-the-valley. Indigenous people in various parts of the world have used many plant extracts containing cardiac glycosides as arrow and ordeal poisons. The ancient Egyptians used squill (Urginea maritime) as a medicine. The Romans employed it as a diuretic, heart tonic, emetic, and rat poison. Digitalis, or foxglove, was mentioned in the year 1250 in the writings of Welsh physicians. Fuchsius described it botanically 300 years later and named it Digitalis purpurea.
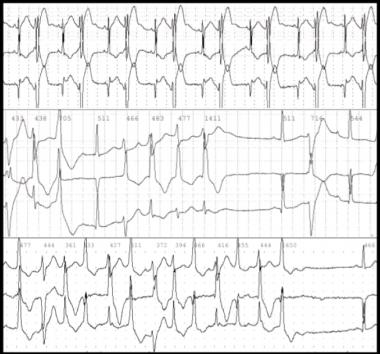
Ecg Digitalis Toxicity
William Withering published his classic account of foxglove and some of its medical uses in 1785, remarking upon his experience with digitalis. He recognized many of the signs of digitalis toxicity, noting, "The foxglove, when given in very large and quickly repeated doses, occasions sickness, vomiting, purging, giddiness, confused vision, objects appearing green or yellow; increased secretion of urine, slow pulses, even as low as 35 in a minute, cold sweats, convulsions, syncope, death." (See Presentation and Workup.)
During the early 20th century, as a result of the work of Cushny, Mackenzie, Lewis, and others, the drug was gradually recognized as specific for treatment of atrial fibrillation. Only subsequently was the value of digitalis for treatment of CHFestablished. Cardiac glycosides enhance cardiac contractility and slow conduction through the atrioventricular (AV) junction by increasing vagal tone. (See Etiology.)
Cardiac glycoside toxicity has been known to result from ingestion of some plants, including yellow oleander (Thevetia peruviana) and foxglove, and a similar toxidrome has been associated with the use of herbal dietary supplements that contain cardiac glycosides.
Digoxin is among the top 50 prescribed drugs in the United States. In 2011, the American Association of Poison Control Centers reported 1601 single exposures to cardiac glycoside drugs. Cardiac glycosides account for 2.6% of toxic plant exposures in the United States. Most of these exposures are in children. (See Epidemiology.)
Digoxin-specific fragment antigen-binding (Fab) antibody fragments have contributed significantly to the improved morbidity and mortality of toxic patients since their approval in 1986 by the US Food and Drug Administration (FDA).
Pathophysiology
Digoxin and other cardiac glycosides cause direct vasoconstriction in the arterial and venous system in vascular smooth muscle. The positive inotropic effect of digitalis has the following 2 components:
-
Direct inhibition of membrane-bound sodium- and potassium-activated adenosine triphosphatase (Na +/K + -ATPase), which leads to an increase in the intracellular concentration of calcium ([Ca 2+]i)
-
Associated increase in a slow inward calcium current (iCa) during the action potential (AP); this current is the result of movement of calcium into the cell, and it contributes to the plateau of the AP
Digitalis glycosides bind specifically to Na+/K+ -ATPase, inhibit its enzymatic activity, and impair active transport of extruding sodium and transport of potassium into the fibers (3:2 ratio). As a result, intracellular sodium ([Na+]i) gradually increases, and a gradual, small decrease in intracellular potassium ([K+]i) occurs.
Cardiac fiber calcium [Ca2+]i is exchanged for extracellular sodium (3:1 ratio) by a transport system that is driven by the concentration gradient for these ions and the transmembrane potential. Increase in [Na+]i is related crucially to the positive inotropic effect of digitalis.
In addition, by a mechanism that is not defined clearly, the increase in [Ca2+]i increases the peak magnitude of iCa; this change parallels the positive inotropic action. The change in iCa is a consequence of the increase in [Ca2+]i and not of the increase in [Na+]i. Thus, more calcium is delivered during the plateau of each AP to activate each contraction.
A fall in intracellular pH accompanies the digoxin-induced increase in [Ca2+] i, which leads to activation of a sodium/hydrogen exchange pump. This results in extrusion of hydrogen, an increase in [Na+]i, and greater inotropy.
The mechanism described assumes that Na+/K+ -ATPase is the pharmacologic receptor for digitalis and that when digitalis binds to these enzymes, it induces a conformational change that decreases active transport of sodium. Digitalis apparently binds to ATPase in a specific and saturable manner, producing a conformational change of the enzyme such that the binding site for digitalis probably is on the external surface of the membrane. Furthermore, the magnitude of the inotropic effect of digitalis is proportional to degree of inhibition of the enzyme.
Digitalis, in therapeutic concentrations, exerts no effect on the contractile proteins or on the interactions between them.
Electrophysiologic effects
The electrophysiological effects of cardiac glycosides include the following :
-
Decreased resting potential (RP) or maximal diastolic potential (MDP), which slows the rate of phase-0 depolarization and conduction velocity
-
Decrease in action potential duration (APD), which results in increased responsiveness of fibers to electrical stimuli
-
Enhancement of automaticity, which results from an increase in the rate of phase 4 depolarization and from delayed after-depolarization
In general, cardiac glycosides slow conduction and increase the refractory period in specialized cardiac conducting tissue by stimulating vagal tone. Digitalis has parasympathetic properties, which include hypersensitization of carotid sinus baroreceptors and stimulation of central vagal nuclei.
Digoxin also appears to have variable effects on sympathetic tone, depending on the specific cardiac tissue involved.
Dosage and toxicity
The therapeutic daily dose of digoxin ranges from 5-15 mcg/kg. The absorption of digoxin tablets is 70-80%; its bioavailability is 95%. The kidney excretes 60-80% of the digoxin dose unchanged.
The onset of action after oral (PO) administration occurs in 30-120 minutes; the onset of action with intravenous (IV) administration occurs in 5-30 minutes. The peak effect with PO dosing is 2-6 hours, and that with IV dosing is 5-30 minutes. Only 1% of the total amount of digoxin in the body is in the serum; of that amount, approximately 25% is protein bound.
Digoxin has a large volume of distribution, being 6-10 L/kg in adults, 10 L/kg in neonates, and as much as 16L/kg in infants and toddlers. At therapeutic levels, the elimination half-life is 36 hours. In acute digoxin intoxication in toddlers and children, the average plasma half-life is 11 hours. With acute intoxication, plasma concentrations extrapolated to time zero are lower in toddlers than in infants and older children because of their increased volume of distribution and clearance.
The lethal dose of digoxin is considered to be 20-50 times the maintenance dose taken at once. In healthy adults, a dose of less than 5 mg seldom causes severe toxicity, but a dose of more than 10 mg is almost always fatal. In the pediatric population, the ingestion of more than 4 mg or 0.3 mg/kg portends serious toxicity. However, plasma concentration does not always correlate with the risk of toxicity.
Digoxin in pregnancy
Digoxin is used widely in the acute management and prophylaxis of fetal paroxysmal supraventricular tachycardia, as well as in rate control of atrial fibrillation. It is an FDA pregnancy category C drug. An increased digoxin dosage may be necessary during pregnancy because of enhanced renal clearance and expanded blood volume.
No series has been published regarding toxicity in the pregnant woman. Digoxin-specific Fab fragments can be used in pregnancy with the caveat that careful monitoring of the fetus must be maintained. Fetal myocardium has a higher resistance to the toxic effects of digitalis.
Dysrhythmias
Alterations in cardiac rate and rhythm from digitalis toxicity may simulate almost every known type of dysrhythmia. Although no dysrhythmia is pathognomonic for digoxin toxicity, toxicity should be suspected when evidence of increased automaticity and depressed conduction is noted. Underlying these dysrhythmias is a complex influence of digitalis on the electrophysiologic properties of the heart through the means already discussed, as well as via the cumulative results of the direct, vagotonic, and antiadrenergic actions of digitalis.
The effects of digoxin vary with the dose and differ depending on the type of cardiac tissue involved. The atria and ventricles exhibit increased automaticity and excitability, resulting in extrasystoles and tachydysrhythmias. Conduction velocity is reduced in myocardial and nodal tissue, resulting in increased PR interval and AV block accompanied by a decrease in the QT interval.
In addition to these effects, the direct effect of digitalis on repolarization often is reflected in the electrocardiogram (ECG) by ST segment and T-wave forces opposite in direction to the major QRS forces. The initial electrophysiologic manifestation of digitalis effects and toxicity usually is mediated by increased vagal tone.
Early in acute intoxication, depression of sinoatrial (SA) or AV nodal function may be reversed by atropine. Subsequent manifestations are the result of direct and vagomimetic actions of the drug on the heart and are not reversed by atropine.
Ectopic rhythms are due to enhanced automaticity, reentry, or both, and may include the following:
-
Nonparoxysmal junctional tachycardia
-
Extrasystole
-
Premature ventricular contractions
-
Ventricular flutter and fibrillation
-
Atrial flutter and fibrillation
-
Bidirectional ventricular tachycardia
Bidirectional ventricular tachycardia is particularly characteristic of severe digitalis toxicity and results from alterations in intraventricular conduction, junctional tachycardia with aberrant intraventricular conduction, or, on rare occasions, alternating ventricular pacemakers.
The following features may also be seen:
-
Depression of the atrial pacemakers, resulting in SA arrest
-
SA block
-
AV block
-
Sinus exit block resulting from depression of normal conduction
-
Nonparoxysmal atrial tachycardia with block
When conduction and the normal pacemaker are both depressed, ectopic pacemakers may take over, producing atrial tachycardia with AV block and nonparoxysmal automatic AV junctional tachycardia. Indeed, AV junctional blocks of varying degrees, alone or with increased ventricular automaticity, are the most common manifestations of digoxin toxicity, occurring in 30-40% of cases. AV dissociation may result from suppression of the dominant pacemaker with escape of a subsidiary pacemaker or inappropriate acceleration of a ventricular pacemaker.
Arrhythmias can cause inadequate tissue perfusion, with resultant central nervous system (CNS) and renal complications, such as the following:
-
Hypoxic seizures
-
Encephalopathies
-
Loss of vasoregulation
-
Acute tubular necrosis
Hyperkalemia is the major electrolytic complication in acute, massive digoxin poisoning. In pediatric patients, hyperkalemia can be a complication of acute toxicity.

Etiology
Clinical digoxin toxicity represents a complex interaction between digoxin and various electrolyte and renal abnormalities. A patient with normal digoxin levels (0.5-2 ng/mL) but renal insufficiency or severe hypokalemia may have more serious cardiotoxicity than a patient with high digoxin levels and no renal or electrolyte disturbances.
Acute overdose or accidental exposure to plants containing cardiac glycosides may cause acute toxicity. Deteriorating renal function, dehydration, electrolyte disturbances, or drug interactions usually precipitate chronic toxicity.
The most common precipitating cause of digitalis intoxication is depletion of potassium stores, which occurs often in patients with heart failure as a result of diuretic therapy and secondary hyperaldosteronism. Dosing errors, especially in infants receiving parenteral digoxin, is a frequent cause of digoxin toxicity and is usually associated with high mortality.
Toxicity may also occur via increased bioavailability. Bioavailability varies depending on the drug formulation. For example, Lanoxin has 25% less bioavailability than Lanoxicaps. Certain antibiotics that suppress intestinal flora may increase absorption of digoxin.
Acute, nontherapeutic overdose—unintentional, suicidal, or homicidal—can cause toxicity. Other causes of digitalis toxicity include the following:
-
Advanced age
-
Myocardial infarction or ischemia
-
Hypothyroidism
-
Hypercalcemia
-
Renal insufficiency
-
Hyperthyroidism
-
Hypoxemia
-
Alkalosis
-
Acidosis - Depresses the Na +/K + ATPase pump and may cause digoxin toxicity
-
Myocardial disease
Both acidosis and myocardial ischemia suppress the Na+/K+ ATPase pump. In addition, myocardial ischemia independently alters myocardial automaticity. Hypothyroid patients are prone to digoxin toxicity secondary to decreased renal excretion and a smaller volume of distribution.
Electrolytes
Hypomagnesemia, hypercalcemia, hypernatremia, hyperkalemia, and hypokalemia can aggravate toxicity. Hypokalemia is usually observed with chronic toxicity or in patients taking diuretics; it reduces the rate of Na+/K+ ATPase pump turnover and exacerbates pump inhibition due to digitalis.
Hyperkalemia is the usual electrolyte abnormality precipitated by digoxin toxicity, primarily in the acute setting. Hyperkalemia may be associated with acute renal failure that subsequently precipitates digoxin toxicity. Chronic digoxin toxicity does not usually cause hyperkalemia. In pediatric patients, hyperkalemia is usually a complication of acute toxicity rather than a cause; however, preexisting hyperkalemia increases the risk of morbidity and mortality.
Medications
Some medications directly increase digoxin plasma levels; other medications alter renal excretion or induce electrolyte abnormalities. Drugs that have been reported to cause digoxin toxicity include the following:
-
Amiloride - May reduce the inotropic response to digoxin
-
Amiodarone - Reduces renal and nonrenal clearance of digoxin and may have additive effects on the heart rate
-
Benzodiazepines (eg, alprazolam, diazepam) - Have been associated with isolated reports of digoxin toxicity
-
Beta-blockers (eg, propranolol, metoprolol, atenolol) - May have additive effects on the heart rate; carvedilol may increase digoxin blood levels in addition to potentiating its effects on the heart rate
-
Calcium channel blockers - Diltiazem and verapamil increase serum digoxin levels; not all calcium channel blockers share this effect
-
Cyclosporine - May increase digoxin levels, possibly due to reduced renal excretion
-
Erythromycin, clarithromycin, and tetracyclines - May increase digoxin levels
-
Propafenone - Increases digoxin level; effects are variable.
-
Quinidine - Increases digoxin level substantially but clinical effect is variable; related drugs, such as hydroxychloroquine and quinine, may also affect levels.
-
Propylthiouracil - May increase digoxin levels by reducing thyroid hormone levels
-
Indomethacin
-
Spironolactone - May interfere with digoxin assays, may directly increase digoxin levels, and may alter renal excretion
-
Hydrochlorothiazide
-
Furosemide and other loop diuretics
-
Triamterene
-
Amphotericin B - May precipitate hypokalemia and subsequent digoxin toxicity
-
Succinylcholine - Increased risk of dysrhythmias has been reported
-
Herb/nutraceutical - Ephedra increases the risk of cardiac stimulation; natural licorice causes sodium and water retention and increases potassium loss
Epidemiology
Approximately 0.4% of all hospital admissions in the United States are related to digitalis toxicity, while about 1.1% of outpatients on digoxin and 10-18% of people in nursing homes develop this toxicity. According to a large study published in 1990, definite digoxin toxicity occurred in 0.8% of patients with heart failure treated with digoxin.
A study by See et al estimated that 5156 emergency department (ED) visits for digoxin toxicity occurred annually in the United States between 2005 and 2010. The study, which used data from the National Electronic Injury Surveillance System—Cooperative Adverse Drug Event Surveillance Project, the National Ambulatory Medical Care Survey, and the National Hospital Ambulatory Medical Care Survey, also estimated that 1% of ED visits for adverse drug events in patients aged 40 years or older resulted from digoxin toxicity, with this figure rising to 3.3% for patients aged 85 years or older.
In 2011, the American Association of Poison Control Centers (AAPCC) reported 1,336 single exposures to plant cardiac glycosides and 1,601 single exposures to drug cardiac glycosides.
The AAPCC reported that the number of digitalis exposures was far less than that of calcium channel blocker toxicities (5,140 cases) or beta-blocker toxicities (10,485 cases). However, the mortality rate from digitalis toxicity was far higher, with 27 deaths reported versus 26 deaths from calcium channel antagonists and 9 deaths attributed to beta-blocker toxicity.
In the United States, hospitalizations for digitalis toxicity declined dramatically from 1991 to 2004. This decline has been attributed to a number of factors, including increased awareness of drug interactions, replacement of digoxin with other drugs and procedures (eg, catheter ablation) for the treatment of heart failure and arrhythmias, and the availability of accurate, rapid radioimmunoassays to monitor drug levels.
Internationally, approximately 2.1% of inpatients are taking digoxin. Of all patients admitted to the hospital, 0.3% develop digoxin toxicity.
Sexual and age-related differences in incidence
Pediatric poisonings from any substance are more common in males than in females. However, for digoxin toxicity, a Netherlands study found no difference in incidence between boys and girls. The adult literature suggests that women may be more susceptible to adverse effects than are men.
Advanced age (>80 y) is an independent risk factor for digitalis toxicity, being associated with increased morbidity and mortality. Older individuals with multiple comorbid conditions have a lower digitalis tolerance than do younger individuals with few or no comorbid conditions.
Manifestations of digitalis toxicity vary depending on age. For instance, ventricular ectopy is most prevalent in older patients; conduction defects and supraventricular ectopic rhythms are most prevalent in younger patients. Children (≤19 y) account for almost 80% of plant exposures and 20% of drug toxicity/poisonings reported to the AAPCC. In most of these cases, the child was younger than 6 years. One study suggests that adolescents are more susceptible to digoxin on a mg/kg basis.
Source emedicine.com
Duc Tin Surgical Clinic
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







