Vesteck scientific advisory board members along with key opinion leaders from around the world have successfully completed the first three in-human cases with the Suture-Tight catheter, a press release reports.
A newly published meta-analysis of individual patient data has found that older patients with symptomatic carotid disease are likely to benefit as much from timely intervention as younger patients. Speaking to Vascular News in light of this key finding, senior author Dominic Howard (Oxford University Hospitals NHS Trust, Oxford, UK) stresses that “vascular surgeons must not turn down symptomatic patients just because of their age”.
Artificial intelligence (AI) models can be developed to predict the risk of postoperative complications after endovascular aneurysm repair (EVAR) with “high accuracy,” research presented at this year’s Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM 2022; 15–18 June, Boston, USA) demonstrated.
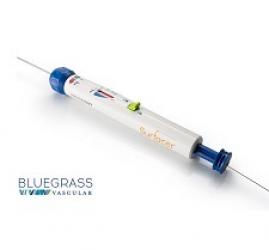
Bluegrass Vascular Technologies recently announced the publication of a paper featured on the cover of the June 2022 issue of the Journal of Vascular Surgery (JVS). The paper reports the use of the company’s Surfacer system to perform an ‘Inside-Out’ procedure and obtain central venous access in a haemodialysis patient presenting with severely symptomatic superior vena cava (SVC) syndrome.
Based on over four years of experience in a single centre, Robert Shahverdyan (Asklepios Klinik Barmbek, Hamburg, Germany) claims that endovascular arteriovenous fistula (endoAVF) creation “keeps future options open,” but is not a replacement for surgical fistula creation.
New data provide “another argument” for the use of intravascular ultrasound (IVUS) in below-the-knee (BTK) interventions. This is according to Michael Lichtenberg (Arnsberg Vascular Center, Arnsberg, Germany), who presented one-year outcomes from the PRESTIGE pilot study at the Leipzig Interventional Course (LINC) 2022 (6–9 June, Leipzig, Germany).
Cagent Vascular has announced the expansion of its product offering to include larger balloons to treat superficial femoral and popliteal arteries in the above-the-knee (ATK) segment.
Shockwave Medical and Genesis MedTech Group announced today that they have successfully obtained approval from China’s National Medical Products Administration (NMPA) to market and sell the Shockwave intravascular lithotripsy (IVL) system with the Shockwave C2 coronary IVL catheters and the Shockwave M5 and S4 peripheral IVL catheters in China.
New long-term data from the SAFE-PAD (Safety assessment of femoropopliteal endovascular treatment with paclitaxel-coated devices) study were presented today as late-breaking clinical research at the Society for Cardiovascular Angiography & Interventions (SCAI) 2022 Scientific Sessions (19–22 May, Atlanta, USA). The analysis found no meaningful difference in survival between patients treated with a paclitaxel drug-coated device and those treated with a non-drug-coated device for up to six years after the index procedure, regardless of the patient’s mortality risk and device type.
The Society for Cardiovascular Angiography & Interventions (SCAI) has released a position statement outlining competencies for endovascular specialists who provide care for chronic limb-threatening ischemia (CLTI).
iVascular has announced the initiation of its first trial with the new generation covered stent iCover—the iliCo study. The study has the objective of evaluating the safety and effectiveness of iCover for the treatment of de novo aorto-iliac atherosclerotic lesions in patients with symptomatic arteriopathy of the lower limbs.
Medtronic announced new randomised controlled data demonstrating the sustained and superior performance of IN.PACT AV drug-coated balloon (DCB) compared to percutaneous transluminal angioplasty (PTA) through 36 months, with no difference in mortality. The data was presented as a Podium 1st at the 2022 Charing Cross (CX) Symposium in London, UK.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389