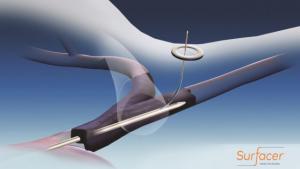
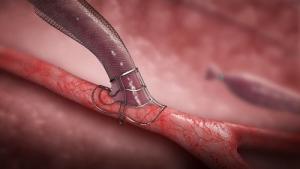
Bluegrass Vascular Technologies has announced the publication of positive results associated with a clinical study involving the use of the Surfacer Inside-Out access catheter system in the peer-reviewed American Journal of Kidney Diseases.
Described as “deliberately challenging”, the new time-to-treatment targets published by the Vascular Society of Great Britain and Ireland (VSGBI), as part of the Peripheral Arterial Disease Quality Improvement Framework, have prompted a number of different measures in the UK for the treatment of patients with chronic limb-threatening ischaemia (CLTI).
A pivotal, multicentre clinical trial is exploring the use of an Impella heart pump (Abiomed) to unload the left ventricle for 30 minutes prior to coronary revascularisation. The aim of the trial is to test the hypothesis that unloading the left ventricle for 30 minutes prior to revascularisation reduces myocardial damage from a myocardial infarction and, as a result, leads to a reduced risk of a patient developing heart failure. The enrolment of the first patient in the STEMI DTU (ST-elevation myocardial infarction door-to-unloading) randomised trial enrolment took place at Spectrum Health (Grand Rapids, USA).
Laminate Medical Technologies (Laminate) has announced the completion enrolment of the VALUE study for the VasQ External Support. The postmarket study enrolled 80 patients (50 upper arm and 30 forearm fistulas) for sites across Germany, France, Spain, and the UK and will be followed for one year. The study is the first prospective study to assess the use of the device in the forearm. The forearm fistula carries a higher risk of primary failure relative to upper arm fistulas but is preferred by surgeons as it preserves more options for the patient’s future access needs.
George Dangas (Mount Sinai Hospital, New York, USA) told delegates in a late-breaking trial session at the American Heart Association Scientific Sessions (AHA 2019; 16–18 November, Philadelphia, USA) that “in patients without an established indication for oral anticoagulation after successful transcatheter aortic valve implantation (TAVI), a treatment strategy including rivaroxaban at a dose of 10mg daily was associated with a higher risk of death or thromboembolic complications and a higher risk of bleeding than an antiplatelet-based strategy”.
A significant milestone has been reached for a landmark study into the use of short duration dual anti-platelet therapy (DAPT) in high-bleeding-risk (HBR) patients following stenting procedures, with patient recruitment just completed.
InnovHeart—a developer of novel transcatheter mitral valve replacement (TMVR) systems—has appointed medical device entrepreneur J Brent Ratz as nonexecutive independent member of its Board of Directors. A press release reports that Ratz has more than 19 years of experience in the medical device industry. He is currently cofounder and managing director of inQB8 Medical Technologies and serves on the Board of Access Vascular.
The European Association of Cardio-Thoracic Surgery (EACTS) has withdrawn its support of the left main recommendations of the society’s joint guidelines with the European Society of Cardiology (ESC) on myocardial revascularisation following an investigation into the EXCEL trial by the BBC current affairs programme Newsnight. The programme has cast doubt upon the conclusions drawn by EXCEL trial investigators.
nnovHeart—a developer of novel transcatheter mitral valve replacement (TMVR) systems—has appointed medical device entrepreneur J Brent Ratz as nonexecutive independent member of its Board of Directors. A press release reports that Ratz has more than 19 years of experience in the medical device industry. He is currently cofounder and managing director of inQB8 Medical Technologies and serves on the Board of Access Vascular.
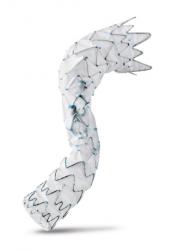
CryoLife recently announced that it has received CE mark for the E-nside thoracoabdominal aortic aneurysm (TAAA) multibranch stent graft system for the endovascular treatment of TAAAs.
A wrist-based optical blood-pressure monitor has the potential to replace cuff monitoring systems for ambulatory blood pressure, suggests a study in the journal Blood Pressure Monitoring. A press release from Aktiia, the Swiss-based company manufacturing the device, states that the novel system uses the same sensors to measure heart rate as those used in wearable devices.
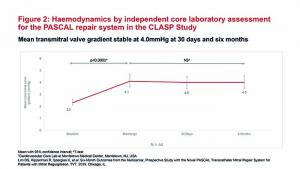
Data from the CLASP study
The CLASP study enrolled 62 patients with MR grade 3+ or 4+ (56% functional, 36% degenerative, and 8% with mixed aetiology). The patients had a mean age of 76.5 years and 51.6% were in New York Heart Association (NYHA) class III or IV.1

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389