Medtronic has announced that it will begin enrolment in a pilot study evaluating the safety and efficacy of the Symplicity Spyral renal denervation (RDN) system using a targeted procedural approach with a reduced number of radio frequency (RF) ablations. Set to enrol 50 patients at up to 15 sites in the USA and Europe, the single-arm study is designed to focus on ablating the distal main renal artery and primary branches to lower blood pressure utilising a simplified procedural approach.
According to the findings of a European multicentre retrospective analysis, the use of drug-coated balloons (DCBs) for the treatment of symptomatic central venous stenosis in dialysis patients has been demonstrated as safe. Presented at the Leipzig Interventional Course 2020 (LINC; 28–31 January, Leipzig, Germany), the investigation adds to a growing evidence-base for DCBs as a potential treatment for patients in dialysis access.
Laminate Medical Technologies has announced the completion of enrolment into the VasQ external support US pivotal study. The study was conducted at 17 sites across the USA and prospectively enrolled 144 male and female patients in need of an arteriovenous fistula (AVF) for haemodialysis. Both brachiocephalic and radiocephalic AVF patients were included in the study. The patients will be followed for two years with the primary endpoint of primary patency analysed at six months.
Merit Medical Systems has announced that both its EmboCube (syringe-loaded embolisation gelatin foam) and Torpedo (uniform, preshaped gelatin foam loaded into a cartridge with optional blunt stylet) devices are now US Food and Drug Administration (FDA)-indicated for the embolisation of blood vessels to occlude blood flow, which helps control bleeding and haemorrhaging in the peripheral vasculature.
Intact Vascular has announced US Food and Drug Administration (FDA) pre-market approval (PMA) for the expansion of its Tack Endovascular System (6F) portfolio. The new approved device size is indicated for repair of post-angioplasty dissections in superficial femoral and proximal popliteal arteries ranging from 4–8mm reference vessel diameter, allowing treatment of a broader range of vessels compared to the current 6F Tack implant offering.
Profusa has announced research findings that suggest the company’s Lumee oxygen platform may help improve the clinical management of patients with critical limb ischemia (CLI) who are undergoing endovascular revascularisation treatment (EVT). The data, from a recent post-market clinical study called OMNIA (Oxygen monitoring near ischaemic areas), were detailed in a series of presentations at the 2020 Leipzig Interventional Course (LINC; 28–31 January, Leipzig, Germany).
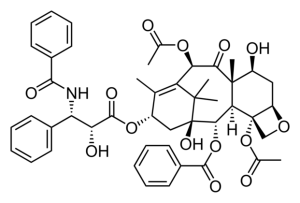
There is no sign of increased all-cause mortality following the use of paclitaxel-coated devices for the treatment of symptomatic peripheral arterial occlusive disease, a new study based on German claims data attests in the European Journal of Vascular and Endovascular Surgery.
Abbott has announced US Food and Drug Administration (FDA) approval of a new alternative surgical technique for Abbott’s HeartMate 3 heart pump that the company says will allow more advanced heart failure patients to avoid open heart surgery. The heart pump can now be implanted through an incision in the chest wall versus open heart surgery. The new, less invasive approach is designed to provide surgeons with a choice in surgical method for patients receiving the HeartMate 3 left ventricular assist device (LVAD), according to a statement from the company.
A retrospective registry comparing four self-expanding transcatheter heart valves has demonstrated good haemodynamic performance in small aortic annuli, with low post-procedure gradients, large orifice areas, and a low incidence of severe patient–prosthetic mismatch (PPM).
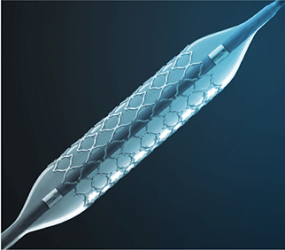
Proximo Medical has announced a partnership in select US markets with CeloNova Biosciences, supplier of the COBRA PzF nanocoated coronary stent (NCS).
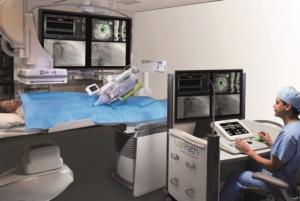
Corindus has announced the completion of the first multicity, transcontinental percutaneous coronary intervention (PCI) simulations in the USA over three network connection types: 5G wireless, dedicated fibre, and commercial public internet networks. A press release reports that Ryan Madder (Frederik Meijer Heart & Vascular Institute, Grand Rapids, USA) successfully completed 36 cases in the same day between Waltham and New York City, and between Waltham and San Francisco (all USA).
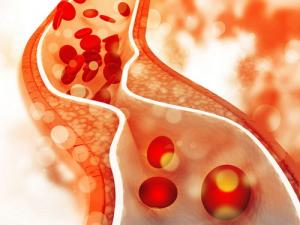
Peripheral artery disease develops silently, narrowing blood vessels for decades until the supply of nutrients and oxygen falls low enough to cause cramps and leg pain.
Initially, the body may form smaller blood vessels around the blocked ones to reroute some blood.
Once the condition advances, those who experience it can develop severe leg cramps when they walk a lot, but that the pain disappears when they sit and the demand for nutrients and oxygen decreases.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389