Fist Assist Devices has just announced completion of the FACT trial, which evaluated the use of an intermittent pneumatic compression device, model FA-1, to promote vein dilation in patients with kidney disease. The trial’s results suggest the device could enable an increase in the creation of functional arteriovenous fistulas by enlarging superficial veins. The trial also demonstrates the device’s safety in this patient population.
Terumo Aortic has announced that the US Food and Drug Administration (FDA) has granted approval of its RelayPro thoracic stent-graft system for sale in the United States for the treatment of patients with thoracic aortic aneurysms (TAA) and penetrating atherosclerotic ulcers (PAUs).
Abbott has announced that it has received US Food and Drug Administration (FDA) clearance for its latest optical coherence tomography (OCT) imaging platform powered by the company’s new Ultreon Software. This innovative imaging software combines OCT with artificial intelligence (AI) to provide physicians an enhanced, comprehensive view of coronary blood flow and blockages to assist physician decision-making and provide the best pathway for treatment.
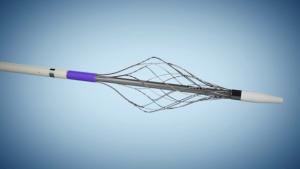
Artio Medical announced today it has completed enrolment in its first-in-human study evaluating the Amplifi vein dilation system. In the study, five patients were treated by Adrian Ebner, head of the Cardiovascular Department at Sanatorio Italiano Hospital in Asunción, Paraguay.
Percutaneous Deep Vein Arterialisation (pDVA) with the LimFlow System offers a cost-effective and high-value alternative to traditional therapies or amputation, according to a recent study published in the Journal of Critical Limb Ischemia.
“There appears to be heightened risk of major amputation after use of paclitaxel-coated balloons [PCBs] in the peripheral arteries,” findings from a systematic review and meta-analysis of randomised controlled trials (RCTs) published in the European Journal of Vascular and Endovascular Surgery (EJVES) this week suggest. However, the authors clarify that the level of evidence is graded moderate, not high, due to scarce events in some studies.
A large, single-centre retrospective study has revealed the risk of acute kidney injury (AKI) following pharmacomechanical thrombolysis (PMT) for lower extremity deep vein thrombosis (DVT) is as high as 22%.
A population-based study from 2013 to 2015 in Germany has found that nearly one fifth of patients with peripheral arterial disease (PAD) did not receive guideline-based vascular diagnostics three months before incidence amputation. Writing online in the European Journal of Vascular and Endovascular Surgery (EJVES), authors Kristina Hagenström (University Medical Center Hamburg-Eppendorf, Hamburg, Germany) and colleagues say this “reflects an underuse of health services”. In addition, the researchers report that, in one third of patients who did not receive vascular surgery, major amputation “probably could have been avoided”.
Endologix today announced the company’s ChEVAS (chimney endovascular aneurysm sealing) system has been granted a Breakthrough Device designation from the US Food and Drug Administration (FDA). The ChEVAS system is an investigational endovascular abdominal aortic aneurysm (AAA)sealing therapy designed to combine the Nellix3.5 endograft with parallel visceral stents to enable treatment of patients with juxtarenal, pararenal, and suprarenal AAA.
Surmodics recently announced that it has acquired privately-held Vetex Medical Limited. The Galway, Ireland-based medical device developer and manufacturer has focused exclusively on venous clot removal solutions. The transaction expands Surmodics’ thrombectomy portfolio with a second US Food and Drug Administration (FDA) 510(k)-cleared device, the ReVene thrombectomy catheter.
A systematic review and meta-analysis has demonstrated that patients undergoing carotid interventions after thrombolysis have a higher risk of periprocedural hazards, compared with those patients who did not have prior thrombolysis. Authors Stavros K Kakkos (University Hospital of Patras, Patras, Greece) and colleagues report their findings in an article published online in the European Journal of Vascular and Endovascular Surgery (EJVES).
Vasorum Limited has announced the appointment of Bob Smouse as a non-executive director.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389