February 2020 brings another paclitaxel device meta-analysis of randomised controlled trials in chronic limb-threatening ischaemia (CLTI) patients. Krystal Dinh (Westmead Hospital, Sydney, Australia) et alreport online ahead of print that the risk of all-cause mortality after treatment with paclitaxel-coated devices versus uncoated controls in patients with CLTI in the Journal of Endovascular Therapy (JEVT) very differently than did Katsanos and colleagues earlier this year. In fact, Dinh et al, due to the clear benefit and no suggestion of a link between the use of paclitaxel-coated devices and mortality, “recommend their continued use in this high-risk patient population”.
Robert Shahverdyan, head of the Vascular Access Center at Asklepios Klinik Barmbek, Hamburg, Germany, has recently published a retrospective analysis of his first 32 consecutive radiocephalic (forearm) VasQ External Support procedures in the Journal of Vascular Access. The paper demonstrated that using VasQ as standard of care for radiocephalic fistulas resulted in a significant improvement in his centre’s primary fistula failure and longer-term secondary patency rates with less repeat interventions when compared to historical controls.
Cerus Endovascular has received CE mark approval for its lead product, the Contour Neurovascular System, for the treatment of intracranial aneurysms. The system incorporates a fine mesh braid that is deployed across the neck of the aneurysm sac and provides a combination of flow diversion and flow disruption through a single device implant. According to the company’s press release, commercial sales, via a controlled market release across the European Union (EU), are expected to begin during the second quarter of 2020.
Preliminary experience with left subclavian artery (LSA) branched devices indicates low morbidity and stroke rates with high patency rates. This was the conclusion of Gustavo Oderich (Mayo Clinic, Rochester, USA) at this year’s Controversies and Updates in Vascular Surgery annual meeting (CACVS; 22–25 January, Paris, France), in a presentation on whether or not we need LSA branched endografts.
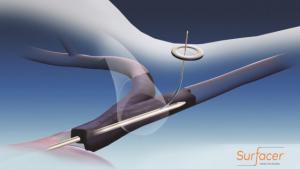
Bluegrass Vascular Technologies (Bluegrass Vascular) announced today that the US Food and Drug Administration (FDA) has granted a de novoclassification order for its Surfacer Inside-Out access catheter system. The Surfacer system is intended to obtain central venous access to facilitate catheter insertion into the central venous system for patients with upper body venous occlusions or other conditions that preclude central venous access by conventional methods. The Surfacer system employs a novel Inside-Out approach.
Gregg W Stone (Icahn School of Medicine, Mount Sinai, New York, USA) addressed the EXCEL controversy today at the Joint Interventional Meeting (JIM 2020; 13–15 February, Milan, Italy), stipulating that although percutaneous coronary intervention (PCI) has early advantages and coronary artery bypass graft (CABG) confers late advantages, in the long term there are no significant major differences in long-term survival, major adverse cardiovascular events (MACE: death, myocardial infarction[MI], or stroke), or quality of life between the two procedures.
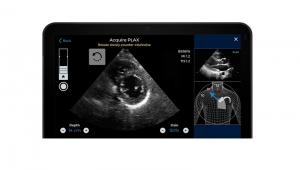
The US Food and Drug Administration (FDA) has authorised marketing of software to assist in the acquisition of cardiac ultrasound, or echocardiography, images. The software, called Caption Guidance (Caption Health), is an accessory to compatible diagnostic ultrasound systems and uses artificial intelligence (AI) to help the user capture images of a patient’s heart that are of acceptable diagnostic quality.
Biotronik has announced CE mark certification for the Orsiro Mission drug-eluting stent (DES) system. According to a company statement, the next generation of the ultrathin strut Orsiro DES provides higher deliverability than other contemporary stents. Orsiro Mission is now available in CE mark countries.
One of the novel applications of the Altura Endograft System is use in the revision of previously placed endovascular grafts or previous abdominal aortic surgery. Jörg Tessarek (Bonifatius Hospital Lingen, Lingen, Germany) has performed a number of such cases, and will be presenting data on his series at the Leipzig Interventional Course (LINC; 28–31 January, Leipzig, Germany). Here, Tessarek discusses the benefits of Altura in this type of revision surgery, details a notable case, and finally considers how this novel application of Altura might be of interest to the wider vascular community.
What are the main challenges of relining aneurysms that have been treated with other devices or by open repair in the past, and which are now failing?
Rist Neurovascular recently announced that it has received US Food and Drug Administration (FDA) 510(k) clearance to market the Rist Cath Radial Access Long Sheath (Rist Cath) for the introduction of interventional devices into the peripheral, coronary, and neurovascular system.
XableCath has announced that its XableCath Crossing catheters have received CE mark for peripheral use. Its crossing devices will be available for sale in Europe in two versions, a blunt tip and an abrasion tip, in a variety of diameters and lengths. XableCath received US Food and Drug Administration (FDA) clearance for its crossing devices last year.
At the Leipzig Interventional Course 2020 (LINC; 28–31 January, Leipzig, Germany), Alvimedica held a Lunch Symopsium in which they introduced a new in-stent treatment of superficial femoral artery (SFA) lesions—Nitides—a technology based on the sustained release of Sirolimus by a SX polymer-free platform.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389