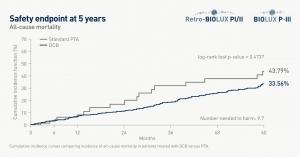
New long-term data presented at the 2022 Charing Cross (CX) Symposium (26–28 April, London, UK) demonstrate the safety of Biotronik’s Passeo-18 Lux paclitaxel drug-coated balloon (DCB) for the treatment of peripheral arterial disease (PAD) in infrainguinal arteries.
The preliminary results from a clinical trial assessing a restorative haemodialysis access graft have shown promising early puncturing, patency and safety data. These findings from an ongoing first-in-human study of the Axess graft (Xeltis) are being presented for the first time by trial investigator Matteo Tozzi (University of Insubria, Varese, Italy) today at the Charing Cross Symposium (CX 2022; 26–28 April, London, UK and virtual).
Cleaning and low-level disinfection (LLD) effective against bloodborne pathogens are safe and sufficient procedures for disinfecting ultrasound transducers used in percutaneous procedures—this is according to an intersocietal position statement recently issued by the American Institute of Ultrasound in Medicine (AIUM).
The third-generation Anaconda endograft (Terumo Aortic) provides “high technical success” and “satisfactory” five-year aneurysm exclusion and clinical success rates, a French multicentre prospective observational study (EPI-ANA-01) has found.
When a modified surgical technique was used to gently remove sections of leg veins used in coronary artery bypass surgery, the grafts were less likely to become blocked and fewer people had a recurrence of heart-related chest pain, according to new research published in Circulation.
Medical management of type B aortic intramural haematomas is associated with low early mortality but a 19% risk of aortic-related intervention—primarily for proximal descending thoracic aneurysms, researchers from the Mayo Clinic in Rochester, USA, have found.
Poverty and Black race were associated with higher rates of lower leg amputation among people with peripheral arterial disease (PAD) who live in metropolitan areas, according to new research published today in a special issue of the Journal of the American Heart Association (JAHA), an open access journal of the American Heart Association (AHA).
Shape Memory Medical has announced the initiation of AAA-SHAPE Netherlands, the company’s prospective, multicentre early feasibility study of the Impede-FX RapidFill device when used for abdominal aortic aneurysm (AAA) sac management during elective endovascular aneurysm repair (EVAR). The Dutch study’s first procedure was performed by Michel Reijnen, vascular surgeon at Rijnstate Hospital in Arnhem, The Netherlands.
The Janssen Pharmaceutical Companies of Johnson & Johnson today announced that the US Food and Drug Administration (FDA) has approved an expanded peripheral arterial disease (PAD) indication for the Xarelto (rivaroxaban) vascular dose (2.5mg twice daily plus aspirin 100mg once daily) to include patients following recent lower-extremity revascularisation (LER) due to symptomatic PAD.
W L Gore & Associates (Gore) has announced that five-year results from the US prospective, multicentre study (n=63) evaluating endovascular repair of iliac aneurysms using the Gore Excluder iliac branch endoprosthesis (IBE) were presented at the Society for Vascular Surgery’s Vascular Annual Meeting (SVS VAM 2021; 18–21 August, San Diego, USA and online). Results of the study confirmed the safety, efficacy, and durability of the IBE for treatment of aortoiliac and iliac artery aneurysms.
MedAlliance has announced completion of patient enrolment in the PRISTINE clinical trial with the Selution SLR 018 drug-eluting balloon (DEB) for the treatment of patients with below-the-knee (BTK) disease. According to a MedAlliance press release, Selution SLR is the first DEB to be awarded Breakthrough Device designation by the US Food and Drug Administration (FDA).

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389