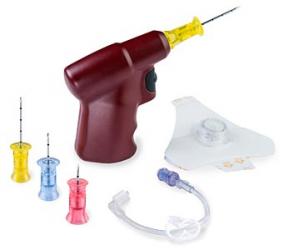
Penumbra today announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the latest iteration of the Indigo aspiration system, Lightning 12.
Teleflex has announced it has received 510(k) clearance from the US Food and Drug Administration to expand the indications for use of the Arrow EZ-IO intraosseous vascular access system. This device can be used when intravenous access is difficult or impossible to obtain in emergent, urgent, or medically necessary cases.
In octogenarians with chronic limb-threatening ischaemia (CLTI), researchers found a one-year mortality rate of 32% after revascularisation, which was significantly higher than in non-octogenarians. Amputation rates were comparable between both age groups.
Biosensors announced today the enrolment of the first patient in REFORM—a prospective, randomised, non-inferiority trial to determine the safety and efficacy of the Biolimus A9 drug coated balloon for the treatment of in-stent restenosis: first-in-man trial. This trial is targeting CE-mark approval for the Biolimus A9 drug coated balloon (DCB) and will include 34 sites in Germany, Italy, Ireland, Spain, the UK and South Korea.
A recent study, published online on 3 August in the European Journal of Vascular and Endovascular Surgery (EJVES), found that regional anaesthesia alone is reasonable for major lower extremity amputation (LEAMP) in high-risk patients, and may initiate a more efficacious enhanced recovery programme than general anaesthesia.
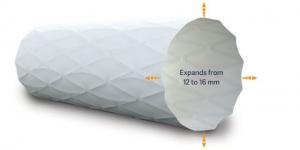
Medtronic today announced the start of a prospective, observational, global, multicentre, real-world, post-market study to evaluate the safety and effectiveness of the Valiant Navion thoracic stent graft system in the treatment of thoracic aortic dissection. The first patient procedure in the DISSECT-N study was performed at Northwell Health in New York, USA by Derek Brinster, director of Aortic Surgery.
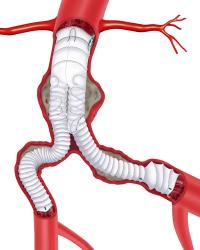
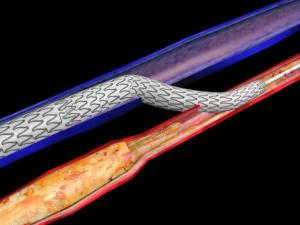
The third-generation Anaconda endograft (Terumo Aortic) provides “high technical success” and “satisfactory” five-year aneurysm exclusion and clinical success rates, a French multicentre prospective observational study (EPI-ANA-01) has found.
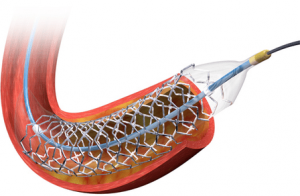
Mid-term data continue to show non-inferiority of resting instantaneous wave-free ratio (iFR) when tested against hyperaemic fractional flow reserve (FFR), said Javier Escaned (Hospital Clinico San Carlos, Madrid, Spain) who reported a pooled, nearly 4,500 patient-level analysis of DEFINE FLAIR and iFR SWEDEHEART trials during the PCR e-Course 2020.
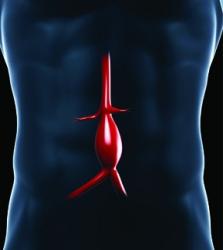
A new study by researchers at the University of Maryland School of Medicine (UMSOM; Baltimore, USA) found that patients with an abdominal aortic aneurysm (AAA) received no benefits from taking a common antibiotic drug to reduce inflammation.
LimFlow SA today announced publication of positive two-year data from the ALPS registry of the LimFlow percutaneous deep vein arterialisation (pDVA) system. Results were published online yesterday in the Journal of Endovascular Therapy and will also appear in the August print issue of the journal.
A study published in Circulation: Cardiovascular Interventions shows that robotic percutaneous coronary intervention with the CorPath GRX system (Corindus Vascular Robotics, Siemens Healthineers) reduces patient exposure to radiation by 20% compared to manual PCI with no increase in fluoroscopy time or contrast use.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389