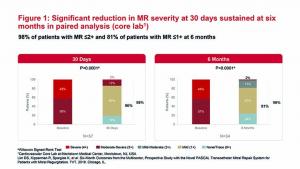
LimFlow SA today announced the presentation of positive six-month data from the full patient cohort in its PROMISE I early feasibility study of the LimFlow percutaneous deep vein arterialisation system. Results were presented on the podium at the 2019 Vascular InterVentional Advances conference (VIVA; 4–7 November, Las Vegas, USA) by Daniel Clair, PROMISE II principal investigator and chair of the department of surgery at the University of South Carolina (USC) and Palmetto Health-USC Medical Group.
It was announced today that results of the TANGO low-dose cohort compared to controls are positive, both for primary and secondary endpoints. These latest findings were presented by Ehrin Armstrong at the 2019 Vascular Interventional Advances conference (VIVA) in Las Vegas, USA (4–7 November). According to Armstrong, perivascular temsirolimus is being developed as a “viable alternative” to prevent restenosis after below-the-knee (BTK) peripheral artery interventions, and TANGO high-dose data is “forthcoming”.
Medtronic today announced Shonin approval from the Ministry of Health, Labour and Welfare (MHLW) and the launch of the Valiant Navion thoracic stent graft system in Japan for the minimally invasive repair of thoracic descending aortic aneurysms (TAA) and complicated type B aortic dissections (TBAD). The news marks the third major geographical launch of the Valiant Navion, following US Food and Drug Administration (FDA) and CE mark approvals in late 2018.
The Solaris (Scitech Medical) is a flexible self-expanding endograft, comprised of a thin multi-direction durable electrospinning PTFE membrane encapsulating a Nitinol stent structure. The device has been engineered to effectively cover and instantaneously seal off diseased tissue with a higher multidirectional resistance force, providing an endoluminal bypass option for physicians faced with complex lesions.
There is a growing need for wound care amid higher rates of chronic wounds—somewhere in the vicinity of five to seven million in the United States per year—at a cost level in excess of US$25 billion, Venita Chandra (Stanford University School of Medicine, Stanford, California, USA) told the America College of Wound Healing and Tissue Repair (ACWHTR; 11–12 October 2019, Chicago, USA).
Clinical data presented at the 2019 European Cardio-Thoracic Surgery (EACTS) annual meeting (3-5 October, Lisbon, Portugal), from two prospective trials of the VEST external stent for vein grafts during coronary artery bypass surgery (CABG), showed low major adverse cardiac and cerebrovascular events (MACCE) rates up to three years, excellent patency rates and a significant reduction in the progression of vein graft disease.
Philips has received US Food and Drug Administration (FDA) approval for two Stellarex 0.035” low-dose (200mm and 150mm) drug-coated balloons for the treatment of de novo and restenotic lesions in native superficial femoral or popliteal arteries.
Michael R Jaff is to be vice president, clinical affairs, innovation and technology, peripheral interventions of Boston Scientific by January 2020. In this role, a press release reports, Jaff will lead clinical and medical affairs strategies to support the development and commercialisation of the company’s peripheral vascular medical device portfolio, and drive engagement with external stakeholders to advance technologies that deliver strong clinical value and enable patient care.
PQ Bypass recently announced it has received full approval of its investigational device exemption (IDE) trial of the company’s TORUS stent graft, a novel stent graft platform designed for the treatment of peripheral artery disease (PAD) in the superficial femoral artery (SFA).
For patients with emergent large vessel occlusion (LVO) eligible for endovascular therapy, prehospital triage to a more distant comprehensive stroke centre (CSC) compared with a closer primary stroke centre (PSC) was associated with significantly shorter time to thrombectomy, better clinical outcomes and no delay to alteplase. Within a matched-pairs model, this association held true for the entire cohort, report Mahesh V Jayaraman, Ryan A McTaggart and their colleagues from Warren Alpert Medical School of Brown University, Providence, USA.
At the 2019 Transcatheter Cardiovascular Therapeutics (TCT) meeting (25–29 September, San Francisco, USA), InterVene won the TCT-Shark Tank Innovation Competition for its novel endovenous system (BlueLeaf) for forming valves in patients with deep vein reflux and/or chronic venous insufficiency. The award, which is supported by the Jon DeHaan Foundation, means that the company receives $200,000 to further develop their device.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389