Insera Therapeutics has announced that the first two ischaemic stroke patients have been treated with its flagship cyclic aspiration system, CLEAR. The company received CE mark approval for its flagship product, the CLEAR Aspiration System, in March 2019.
In the largest prospective analysis of cerebrospinal fluid (CSF) in patients undergoing endovascular thoracoabdominal aneurysm repair (TAAA) to date, permanent paraplegia was found to be associated with shedding of bound ADVS-1 from the parenchymal cord into CSF and disruption of the blood-spinal cord barrier, which in turn leads to cord oedema and a leucocyte infiltration.
Abiomed has announced the results of PROTECT III, an ongoing, prospective, single-arm US Food and Drug Administration (FDA) post-market approval (PMA) study for Impella 2.5 and Impella CP in high-risk percutaneous coronary intervention (PCI). PROTECT III follows the PROTECT II randomised controlled trial.
According to a press release, AngioDynamics has acquired Eximo Medical and its proprietary 355nm wavelength laser-technology platform for US$46 million in up-front consideration with up to US$20 million of contingent consideration related to certain technical and revenue milestones. The transaction is being funded exclusively through the use of cash on hand.
ONYX ONE, the first randomised trial to compare a durable polymer drug-eluting stent to a polymer-free drug-coated stent in patients at high risk of bleeding and treated with one-month dual antiplatelet therapy (DAPT), has found that both are clinically safe and effective.
AtriCure has entered into a definitive agreement to acquire SentreHEART, which developed the LARIAT device for left atrial appendage closure in patients with atrial fibrillation. A press release reports that the transaction consideration consists of an upfront payment of approximately US$40 million in cash and AtriCure common stock, plus additional contingent consideration based on the achievement of certain clinical and reimbursement milestones
Intact Vascular has announced the positive one-year results of its Tack Optimised Balloon Angioplasty (TOBA) III clinical trial, successfully achieving both primary and secondary endpoints. Marianne Brodmann, head of the Clinical Division of Angiology, Medical University of Graz, Austria and principal investigator of the TOBA III trial, presented the data during the high impact clinical research session of the 31st Transcatheter Cardiovascular Therapeutics (TCT) meeting (25–29 September, San Francisco, USA), the annual scientific symposium of the Cardiovascular Research Foundation.
Terumo Aortic has announced the completion of enrolment of the RelayPro pivotal study in the USA. RelayPro is a low profile, next generation thoracic stent graft device designed to expand the treatment of thoracic endovascular aortic repair (TEVAR) to patients with smaller access vessels.
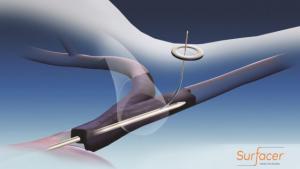
Bluegrass Vascular Technologies (Bluegrass Vascular) has announced the publication of positive results associated with the clinical use of the Surfacer Inside-Out access catheter system in the peer-reviewed Journal of Vascular Access (JVA).
A recent study found that being a smoker, having diabetes, and also having a history of coronary heart disease or stroke increases the lifetime risk for PAD by as much as five times that of someone of the same race, age, and sex who does not have those risk factors. Additionally, race is a strong factor in PAD risk, contributing as much as some other health-related factors. The analysis was led by scientists at the Johns Hopkins Bloomberg School of Public Health and recently featured online in the Journal of the American Heart Association. According to the authors, the study is the first to have quantified the lifetime risk of PAD.
The results of AVeNEW, the first level one clinical study dedicated solely to the use of a covered stent designed to treat stenosis in the arteriovenous (AV) fistula access circuit, show that the Covera vascular covered stent (Bard) demonstrates “significantly better” target lesion primary patency compared to percutaneous transluminal angioplasty (PTA) at six months, with the difference sustained in the 12-month data, and better access circuit patency was maintained at 12 months.
A recent analysis, with some of the longest follow up to date, suggests that patients undergoing endovascular aneurysm repair (EVAR) may experience a significant long term decrease in renal function. The authors, Edmund R Charles and colleagues at the Leicester Vascular Institute for Health Research (NIHR) Leicester Biomedical Centre (Leicester, UK) argue, “This needs to be taken into account when offering EVAR in younger patients,” and suggest that renal follow up and preservation should be optimised in this patient group.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389