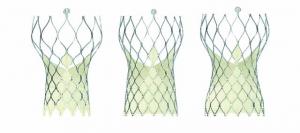
A subanalysis from the SURTAVI trial has found that transcatheter aortic valve implantation (TAVI) with Evolut R in intermediate-risk patients has lower rates of all-cause mortality or disabling stroke than surgical valve replacement 12 months after implantation. Steven J Yakubov (Riverside Methodist Hospital, Columbus, Ohio, USA) presented data from a propensity-score matched comparison at a late-breaking trial session at Cardiovascular Research Technologies (CRT) 2019 (2–5 March, Washington DC, USA). The findings have also been published in the Journal of American College of Cardiology: Cardiovascular Interventions.
In the last 20 years, there have been major developments in the use of endovascular and hybrid haemorrhage control tools, especially in the treatment of ruptured abdominal aortic aneurysm (rAAA).
Innovative Cardiovascular Solutions (ICS) has announced the first clinical use of its next-generation Emblok embolic protection system in patients undergoing transcatheter aortic valve implantation (TAVI) procedures in its European feasibility study. The cases were successfully performed by primary investigator Federico De Marco (Policlinico San Donato, Milan, Italy).
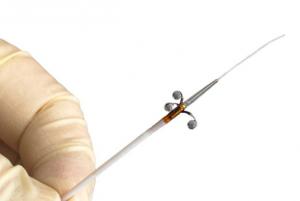
The Peregrine System infusion catheter (Ablative Solutions) has demonstrated 100% procedural success in the Peregrine post-market clinical trial, new data presented during a late-breaking session at the 2019 Cardiovascular Research Technologies (CRT) meeting (2–5 March, Washington, DC, USA) attests.
A pooled data analysis comparing the Cre8 amphilimus-eluting stent (Alvimedica) and the BioFreedom biolimus-eluting stent (Biosensors) demonstrates similar safety and efficacy for the devices, with possible differences in treatment effect for patients with diabetes mellitus. Describing a combined investigation into two multicentre observational studies in Italy in Circulation: Cardiovascular Interventions, the researchers say: “BioFreedom and Cre8 have favourable and comparable safety and efficacy profiles in real-world all-comer patients undergoing percutaneous coronary revascularisation.” Also, they add: “Cre8 might provide a clinical benefit in diabetic patients.”
Medtronic has issued the following statement regarding revised clinical study data:
On 15 February, 2019, Medtronic issued a statement regarding a programming error in the clinical data reporting isolated to the two and three year follow-up periods in our IN.PACT Global post-market study, part of the IN.PACT Admiral clinical programme for the treatment of femoropopliteal artery disease.
The Sapien 3 device (Edwards Lifesciences) can be safely and effectively used in transcatheter aortic valve implantation (TAVI) via the transcarotid approach in patients for whom the transfemoral route is not suitable, according to 30-day results from a multicentre French registry. The authors of the study, published in the JACC: Cardiovascular Interventions, say: “This descriptive study reports the largest contemporary cohort to date of transcarotid TAVI and demonstrates the safety and efficacy of the transcarotid approach for TAVI with the Edwards Sapien 3 transcatheter heart valve.”
The Charing Cross International Symposium (CX) continues its three-year cycle of raising vascular and endovascular controversies in order to challenge the available evidence and be able to reach a consensus after discussion with an expert audience.
The UK’s National Institute of Health and Care Excellence (NICE) has published new guidance recommending UrgoStart wound dressings (Urgo Medical) for treatment of diabetic foot ulcers and venous leg ulcers. In a press release, Urgo Medical states that use of the wound dressing could prevent more than 3,000 diabetes-related amputations.
Medtronic has received US FDA approval of its Resolute drug-eluting stent platform—including the Resolute Onyx and Resolute Integrity—for the treatment of patients with coronary artery disease who have de novo chronic total occlusion. Chronic total occlusions are considered to be more difficult to treat with percutaneous coronary intervention (PCI) than non-chronic total occlusions because of a greater risk of complications.
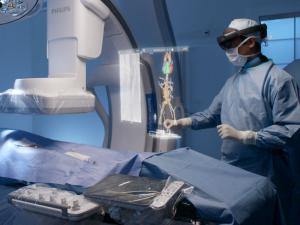
At the MWC (25–26 February, Barcelona, Spain), formerly the Mobile World Congress, Philips unveiled a unique mixed reality concept developed in partnership with Microsoft for the operating room of the future. Based on Philips’ Azurion image-guided therapy platform and Microsoft’s HoloLens 2 holographic computing platform, the companies will showcase novel augmented reality applications for image-guided minimally invasive therapies. The technology allows wearers of the HoloLens 2 headset to access a virtual screen displaying data from the Azurion system, updated in real time, during procedures.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389