One-month dual antiplatelet therapy (DAPT) followed by 12 months of clopidogrel monotherapy leads to a greater reduction in bleeding events following percutaneous coronary intervention (PCI) compared to 12-month dual antiplatelet therapy, without an increase in ischaemia. Hirotoshi Watanabe (Kyoto University, Japan) announced findings on behalf of the STOPDAPT-2 investigators at a late breaking trial session at the American College of Cardiology’s (ACC 2019) 68th Annual Scientific Session (16–18 March) in New Orleans, Louisiana, USA.
The Medicines and Healthcare products Regulatory Agency (MHRA) in the UK and the Food and Drug Administration (FDA) in the USA have both recently issued new statements on the use of paclitaxel-eluting devices. While the FDA release preliminary analysis results from their ongoing review of data, the MHRA announced they have formed an independent Expert Advisory Group, which has now “begun the process of reviewing the available, but highly complex information on these medical devices.” Following preliminary analysis, the FDA meanwhile recommend that “alternative treatment options to paclitaxel-coated balloons and paclitaxel-eluting stents should generally be used until additional analysis of the safety signal has been performed.”
MHRA launches Expert Advisory Group
A new paper looking specifically at mortality has found no difference in deaths between paclitaxel-coated balloons and uncoated balloons when used in the femoropopliteal artery. These data, a pooled analysis of four randomised controlled, multicentre trials, were published in Cardiovascular Interventional Radiology.
A press release reports that preliminary data from the BIOSOLVE-IV registry strengthen the clinical evidence in favour a bioresorbable magnesium scaffold (Magmaris, Biotronik) for de novo coronary artery lesions. Presented as late-breaking clinical data at the 2019 Cardiovascular Research Technologies (CRT) meeting (2–5 March 2019, Washington, DC, USA), 12-month results from the first 600 patients of the registry showed low target lesion failure rates at 5.1% and low incidences of scaffold thrombosis at 0.5%.
Endospan has announced that it has received CE mark for its Nexus stent graft system, to be used for branched endovascular repair in the aortic arch.
Micro Interventional Devices chairman, president and CEO, Michael Whitman, and Oscor president and CEO Thomas Osypka, are pleased to announce a strategic alliance that leverages each company’s unique strengths and capabilities to further develop and commercialize innovative solutions to structural heart disease, specifically, Micro Interventional Devices’ MIA—minimally invasive annuloplasty—technology.
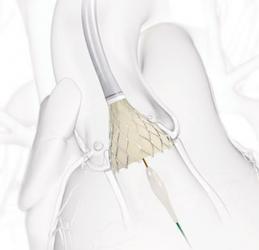
Transcatheter aortic valve implantation (TAVI) with the Evolut system (Medtronic) is as successful as surgical valve replacement in low-risk aortic stenosis patients. Data from the Evolut low risk trial were presented at the American College of Cardiology 68th Annual Scientific Session (ACC 19) and published simultaneously in the New England Journal of Medicine. It compared the minimally invasive Evolut transcatheter aortic valve implantation (TAVI) system to the gold standard of open-heart surgery in characteristically younger, healthier aortic stenosis patients.
Beginning in mid-2019, hospitals performing transcatheter valve repair and replacement will be able to apply for the American College of Cardiology’s (ACC) new Transcatheter Valve Certification. The certification was announced at the ACC Quality Summit in New Orleans.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389