Beacon Tip catheters (Cook Medical) are once again available to physicians in Europe. As of September 2019, the catheters are available in the UK, Ireland, Norway, Sweden, Finland, Denmark, Germany, Poland, the Netherlands, Luxembourg, Belgium, France, Switzerland, Austria, Hungary, Spain, and Italy. Cook is continuing to work on releasing the products to customers in the rest of Europe, the Middle East and Africa in the near future.
A private equity firm, Palatine (Manchester, UK), has announced that it has made a “significant investment” into a UK centre—Veincentre—that provides endovenous laser ablation (EVLA) for the management of varicose veins. The investment, according to Palatine, is the fourth to be made from the firm’s £100m “Impact Fund” that “targets companies with a social and/or environmental impact, alongside industry-standard returns”.
Endologix has announced the investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to commence a new pivotal study to evaluate the safety and effectiveness of the Nellix Chimney EndoVascular Aneurysm Sealing System (chEVAS) for the endovascular treatment of complex abdominal aortic aneurysms (AAA). The company also announced an exclusive distributor agreement with Boston Scientific, to distribute Endologix products in China.
ChEVAS IDE trial
Two-year results from the MOBILE (Marrowstim treatment of limb ischaemia in patients with severe peripheral arterial disease) trial indicate that injecting concentrated bone marrow aspirate (cBMA, Zimmer Biomet), vs. placebo, into patients with critical limb ischaemia is associated with a significant increase in amputation-free survival at two years. However, the results also suggest that diabetic/Rutherford 5 patients do not benefit from the therapy.
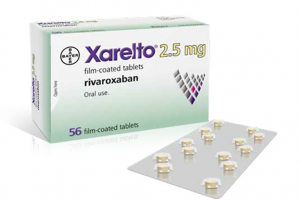
The National Institute for Health and Care Excellence (NICE) in the UK has published a positive draft final appraisal determination recommending the use of rivaroxaban (brand name Xarelto, Bayer) by the National Health Service (NHS) in England. The NICE recommendation sets out a dose of 2.5mg twice daily, combined with 75–100mg aspirin once daily, as an option for preventing atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral arterial disease (PAD) who are at high risk of ischaemic events.
Reflow Medical, Inc announces that the first patients have been enrolled in its DEEPER OUS clinical trial using the Temporary Spur Stent System.
The first patient has been randomised in the TARGET BP I trial at the Piedmont Heart Institute in Atlanta, USA, to investigate the use of the Peregrine System Kit (Ablative Solutions) in renal denervation.
Laminate Medical Technologies has announced the enrolment of their first forearm fistula patients in their US pivotal trial of the VasQ device. VasQ is an implanted blood vessel external support intended to promote maturation and reduce the high primary failure rate of surgically created fistulae for haemodialysis. The forearm fistula is the gold standard for vascular access creation but also has the highest risk of primary failure. The inclusion of the forearm fistula to the US pivotal trial of VasQ represents a significant potential advancement for dialysis patients to help improve their overall care, the company states.
Thanks to the use of robotics in healthcare, many procedures that were once highly invasive are now being performed using minimally invasive techniques, with the added benefits of precision and radiation protection. Jean Fajadet looks at how the technology works, and what it can bring to the cath lab.
Five-year data from the INSPIRATION US investigational device exception (IDE) pivotal trial confirms favourable safety and effectiveness of the INCRAFT device. Presenting the trial at the Society of Vascular Surgery (SVS) Vascular Annual Meeting (VAM; 12–15 June, National Harbor, USA), Michel Makaroun, University of Pittsburgh (Pennsylvania, USA) surmised that the INCRAFT device “offers unique benefits in patients with challenging access anatomy”, while it “may provide a useful option for patients with infrarenal abdominal aortic aneurysms”.
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Centerline Biomedical’s Intra-Operative Positioning System (IOPS), a device used in endovascular procedures to provide 3D visualisation.
A new visualisation method enables more precise catheter navigation through the vascular system. It is being developed at the Fraunhofer Institute for Digital Medicine (MEVIS) in Bremen, Germany, and one of its ambitions is to reduce physician radiation exposure.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389