Four years ago, vascular experts from around the world had a “quixotic quest”, to get all surgeons and providers to agree on the best ways to treat a common and debilitating illness: chronic limb-threatening ischaemia. The goal has come to fruition, the Society for Vascular Surgery (SVS) have announced, with the publication of the new global guidelines, produced by the European Society for Vascular Surgery (ESVS), SVS, and the World Federation of Vascular Societies (WFVS). The guidelines were published in the ESVS’ European Journal of Vascular and Endovascular Surgery as well as in the SVS’ Journal of Vascular Surgery.
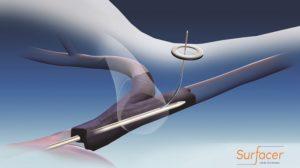
The SAVE-US (Surfacer system to facilitate access in venous occlusions—United States) pivotal trial has completed enrollment. The SAVE-US trial is evaluating the safety and efficacy of the Surfacer Inside-Out access catheter system (Bluegrass Vascular Technologies) in 30 patients across seven US centres, and demonstrated positive clinical results by meeting its primary and secondary endpoints.
Endologix recently announced that the EC Certificate of Conformity (CE mark) for the Nellix endovascular aneurysm sealing system (Nellix system) has been reinstated by GMED, the EU Notified Body for the Nellix system. The reinstatement followed an assessment of clinical evidence.
Do not use paclitaxel drug-coated balloons (DCBs) or drug-eluting stents (DESs) in the routine treatment of patients with intermittent claudication until further notice, a medical device alert (MDA) issued today by the UK Medicines and Healthcare products Regulatory Agency (MHRA) announces, “as the potential mortality risk generally outweighs the benefits”. However, the use of DCBs and DESs may still be considered in patients with critical limb ischaemia, taking National Institute of Care Excellence (NICE) guidance into consideration, in view of their increased risk of restenosis and reduced life expectancy, the MDA accedes.
Investigators of SWEDEPAD have announced the conclusion of their safety committee analysis, which recommends the halted trials resume enrolment.
The US Food and Drug Administration have issued a Class I Recall of the SoloPath (Terumo Medical) ballon-expandable transfemoral system and re-collapsible balloon access system, due to malfunction. A total of 14 reports had flagged related incidents of a specific device malfunction, including two injures. No deaths have been reported, the FDA notes.
Three-year results from the ILLUMENATE Pivotal trial and the ILLUMENATE European randomised controlled trial (EU RCT) have been presented in a late-breaking trial session at the New Cardiovascular Horizons Annual Conference (NCVH; 29–31 May, New Orleans, USA) by S Jay Mathews (Bradenton Cardiology Center, Bradenton, USA). The data demonstrated a significant treatment effect with a high safety profile through three years following treatment with the Stellarex (Philips) sirolimus drug-coated balloon (DCB), with no significant difference in mortality compared to plain percutaneous transluminal angioplasty (PTA).
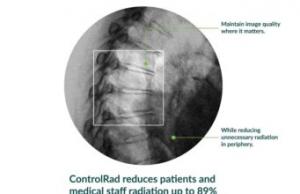
ControlRad has announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for its ControlRad Trace. The company has initiated its commercial launch. The ControlRad Trace is the only technology that can be integrated into existing mobile C-arms to reduce radiation in any fluoroscopic imaging procedure.
A single-centre study of standard, fenestrated and branched endovascular aneurysm repair patients in Nürnberg, Germany, has found that outcomes for women are worse but the reasons are not yet clear, as literature on the topic is lacking. The results and literature review of gender differences in treating abdominal aortic aneurysm were presented by Athanasios Katsargyris (Klinikum Nürnberg, Germany) at the 23rd annual Critical Issues symposium (23–24 May, Liverpool, UK).
The UK’s National Institute for Health and Care Excellence (NICE) has issued draft guidance on abdominal aortic aneurysm (AAA) diagnosis and management. The most notable recommendation within the guideline is related to repairing unruptured aneurysms where the guideline states that patients should not be offered endovascular repair (EVAR) if open surgical repair is suitable.
.1 Evidence supports the case for adopting PICO negative pressure wound dressings for closed surgical incisions in the NHS. They are associated with fewer surgical site infections and seromas compared with standard wound dressings.
1.2 PICO negative pressure wound dressings should be considered as an option for closed surgical incisions in people who are at high risk of developing surgical site infections. Risk factors for surgical site infections are described in section 4.2.
1.3 Cost modelling suggests that PICO negative pressure wound dressings provide extra clinical benefits at a similar overall cost compared with standard wound dressings.
Data presented at EuroPCR today indicates that renal denervation with the Symplicity system (Medtronic) is associated with a reduction in subclinical atrial fibrillation in high-risk patients with hypertension over a median follow-up of two years.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389