CK-MB isoforms
The CK-MB isoenzyme exists as 2 isoforms: CK-MB1 and CK-MB2. Laboratory determination of CK-MB actually represents the simple sum of the isoforms CK-MB1 and CK-MB2. CK-MB2 is the tissue form and initially is released from the myocardium after MI. It is converted peripherally in serum to the CK-MB1 isoform rapidly after symptom onset.
Normally, the tissue CK-MB1 isoform predominates; thus, the CK-MB2/CK-MB1 ratio is typically less than 1. A result is positive if the CK-MB2 is elevated and the ratio is greater than 1.7.
CK-MB2 can be detected in serum within 2-4 hours after onset and peaks at 6-9 hours, making it an early marker for acute MI. Two large studies evaluating its use revealed a sensitivity of 92% at 6 hours after symptom onset, compared with 66% for CK-MB and 79% for myoglobin. [36, 37] The major disadvantage of this assay is that it is relatively labor intensive for the laboratory.
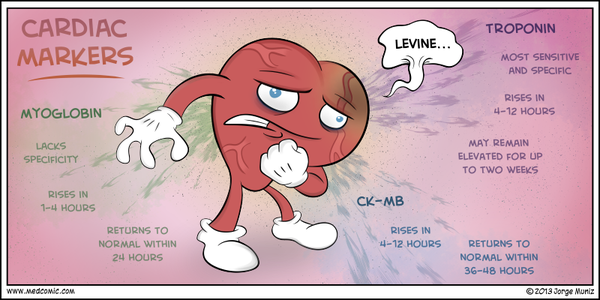
Myoglobin
Myoglobin is a heme protein found in skeletal and cardiac muscle that has attracted considerable interest as an early marker of MI. Its low molecular weight accounts for its early release profile: myoglobin typically rises 2-4 hours after onset of infarction, peaks at 6-12 hours, and returns to normal within 24-36 hours.
Rapid myoglobin assays are available, but overall, they have a lack of cardiospecificity. Serial sampling every 1-2 hours can increase the sensitivity and specificity; a rise of 25-40% over 1-2 hours is strongly suggestive of acute MI. However, in most studies, myoglobin only achieved 90% sensitivity for acute MI, so the negative predictive value of myoglobin is not high enough to exclude the diagnosis of acute MI.
The original studies that evaluated myoglobin used the WHO definition of acute MI that was based on a CK-MB standard. With the adoption of a troponin standard for acute MI in the ACC/ESC definition, the sensitivity of myoglobin for acute MI is substantially reduced. This significantly diminishes its utility, and a number of studies have indicated that contemporary cardiac troponin assays render the use of myoglobin measurements unnecessary.
Testing Strategy
In patients with definite or possible ACS, serial evaluation of cardiac markers is essential to diagnosing acute MI.
The American College of Emergency Physicians (ACEP) recommends 3 different testing strategies for ruling out NSTEMI in the ED. [38] One strategy is to use a single negative CK-MB, TnI, or TnT measured 8-12 hours after symptom onset.
Another strategy is to use negative myoglobin in conjunction with a negative CK-MB mass or negative TnI measured at baseline and at 90 minutes in patients presenting less than 8 hours after symptom onset.
A third approach is to use a negative 2-hour delta CK-MB in conjunction with a negative 2-hour delta TnI in patients presenting less than 8 hours after symptom onset.
Note that ACEP does not specify whether to use the 99th percentile cutoff, the 10% CV cutoff, or the WHO acute MI cutoffs for troponin.
The 90-minute rule-out with myoglobin recommended by ACEP was based on a study that used myoglobin in conjunction with either CK-MB or TnI. The CK-MB/myoglobin protocol yielded a sensitivity of 92% at 90 minutes, and the myoglobin/TnI combination yielded a sensitivity of 97% at 90 minutes.
ACEP acknowledges the relative lack of specificity for myoglobin and that many of the myoglobin studies did not define MI per the ACC/ESC guidelines. Nevertheless, it is difficult to comprehend the ACEP clinical policy that accepts a missed MI rate of 3-8%.
ACEP’s recommendations on the use of delta CK-MB and delta TnI are based on determining the change in the level of TnI or CK-MB on samples drawn 2 hours apart. However, the delta TnI evaluation is partially based on the use of older TnI assays and outdated WHO acute MI cutoffs in a retrospective study. Therefore, ACEP’s recommendation to use a delta TnI in conjunction with a delta CK-MB may not be generalizable to other commercially available troponin assays. Caution must be used when using ACEP’s recommendations in ED patients with chest pain and suspected ACS.
Cardiac Markers in Therapeutic Management
Clinical trials have demonstrated the benefits of using cardiac markers as an indicator for specific therapeutic interventions in ACS. However, this use remains investigational; currently, no validated therapeutic algorithms are based on an isolated positive marker result in the absence of other clinical or ECG findings.
Subgroup analysis of trials with low molecular weight heparin (LMWH) showed a decreased cardiac event rate in patients with a positive result for TnT and who were treated with an LMWH.
Similarly, in the PRISM trial, patients with an elevated TnI who were treated with the glycoprotein (GP) IIb/IIIa inhibitor tirofiban (Aggrastat) demonstrated a significant decrease in cardiac events compared with patients without an elevated TnI level. No significant difference in outcomes was seen in patients without TnI elevations who were treated with tirofiban when compared with placebo.
In the PURSUIT trial, patients who were treated with the GP IIb/IIIa inhibitor eptifibatide (Integrilin) within 6 hours of symptom onset obtained the greatest benefit, and subgroup analysis showed that patients with an elevated troponin level also had better responses to therapy than did those whose troponin result was negative.
Finally, in the TACTICS-TIMI 18 trial, patients with elevations in TnI or TnT had a significant reduction in death, MI, or rehospitalization for ACS within 6 months after being treated with early invasive therapy consisting of aspirin, heparin, tirofiban, and catheterization/revascularization within 4-48 hours. [44, 45] Subset analysis noted that an elevation of CK-MB did not benefit the early invasive group when compared with the conservative management group. However, early invasive therapy did benefit the subgroup of patients with elevated troponin levels but normal CK-MB levels.
These studies suggest that a positive troponin result alone is an independent predictor of high risk for adverse cardiac events, and that therapy with LMWHs and/or GP IIb/IIIa inhibitors appears to confer the most benefit on patients with elevated cardiac troponins levels.
Troponins in CRF
Patients with chronic renal failure (CRF) who are on hemodialysis are at increased risk of coronary artery disease and acute ACS, and cardiovascular disease accounts for about 50% of deaths in these patients. Early studies revealed a high prevalence of elevated cardiac troponin levels in patients with CRF, and especially of TnT. However, the clinical significance of an elevated TnT level is unclear.
Biochemical studies have demonstrated that the troponin elevation originates from the myocardium and is not related to the myopathy associated with renal failure. Yet, patients with CRF frequently have chronic congestive heart failure (CHF) and hypertension, which may independently elevate the troponin level. In addition, data suggest that elevated troponin levels in asymptomatic patients may reflect subclinical microinfarctions that are clinically distinct from ACS.
Large prospective studies have confirmed the association between TnT elevation and cardiac mortality in patients with CRF. The GUSTO IV ACS trial showed that patients with renal insufficiency and an elevated TnT had the highest overall risk of the composite endpoint of death or acute MI, [47] and 2 other prospective studies reported that an elevated TnT—but not TnI—increased the risk of long-term mortality. [48, 49] Whether elevated TnT increases cardiac risk in the short term (ie, 30 d) is unclear, but patients without short-term risk may not require hospitalization and potentially could be managed on an outpatient basis.
It has been suggested that chronically elevated troponin levels represent chronic structural cardiovascular disease, such as prior MI, chronic CHF, or hypertension in the setting of CRF. If true, these patients are at higher cardiac risk compared with the normal, healthy patient population and troponin remains a useful marker in the setting of CRF.
Note that dialysis does not affect TnT or TnI levels; predialysis and postdialysis levels are essentially unchanged. CK-MB, however, is dialyzable, and levels are decreased postdialysis. Therefore, a single elevated TnT level in patients with CRF and possible ACS is nondiagnostic for acute MI in the absence of other findings. Serial determinations are usually required, with a focus on a rise in the troponin level.
Ascertaining whether an elevated troponin in patients with CRF represents true acute MI or a false-positive result can be difficult. In patients with cardiac risk factors who are deemed clinically to be at moderate-high risk for ACS, the prudent approach would be to observe and perform serial cardiac markers over 6-9 hours. In low-risk asymptomatic patients and in the absence of any other findings indicative of ACS, the elevated troponin result is more likely to be false positive for acute MI.
Source emedicine.com
Duc Tin Surgical Clinic
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







