The first-in-human case using the new deep vein thrombosis (DVT) device Vetex thrombectomy catheter (Vetex Medical) has recently been completed in a multicentre study. The device has the potential to reduce hospital stays and costs associated with DVT treatment, a press release announces.
According to Vetex Medical, the Vetex thrombectomy catheter is the first device to combine rotational and grasping action to “quickly and gently” remove large volumes of wall-adherent clot in a single session, without thrombolytic drugs.
The first patient was treated by the study’s principal investigator Stephen Black, Narayan Thulasidasan, and their team at Guy’s and St. Thomas’ NHS Foundation Trust in London, UK.
“The Vetex device was surprisingly effective at removing wall-adherent clot on the first pass and was easy to use in our first procedure. Existing devices can remove fresh thrombus but have difficulty creating a larger lumen through more organised material on the vessel wall,” says Black. “This device shows the potential to start and finish the procedure in one catheter lab session, avoiding intensive care unit/high dependency unit time and a prolonged hospital stay, and thereby saving staff time and hospital costs.”
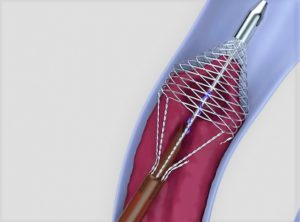
Vetex thrombectomy catheter (Vetex Medical)
The multicentre, non-randomised VETEX trial is a feasibility study of 30 patients with acute iliofemoral DVT treated with the Vetex device, with the primary outcome being procedural success, defined as SIR grade II lysis with freedom from procedural related adverse events.
DVT treatment expert Michael Lichtenberg (Klinikum Hochsauerland GmbH in Arnsberg, Germany), who was involved in the study, comments: “DVT technology is not there today to deliver reliable or reproducible results every time. As physicians, our goal is to evolve treatment to be safer for patients, faster and more cost effective, and the Vetex device shows the potential to deliver all three.”
The goal of the current study is to confirm the design of the device; it offers a purely mechanical approach to removing wall-to-wall clots, avoiding the use of thrombolytic drugs due to the associated bleeding risk, according to Vetex Medical CEO Mark Bruzzi. At present, the Vetex thrombectomy catheter is in the early clinical trial phase.
Vetex Medical plans to seek FDA 510(k) clearance for the device. Eventually, the company also plans to expand the patient population to include not just patients with acute DVT, but also those with subacute DVT without the use of thrombolytic drugs.
Today’s DVT treatment
Currently, anticoagulation is the most widespread therapy for DVT, but interventional treatment has demonstrated the potential for better outcomes in some patients. Interventional DVT options on the market today include the use of thrombolytic drugs to dissolve a clot, with or without the use of a mechanical device, or purely mechanical devices that use fragmentation and/or aspiration to create a core through the clot. Because thrombolytic agents thin the blood, they present a bleeding risk for many patients and can require prolonged hospital stays. Without effective treatment, up to 50% of patients with symptomatic DVT will develop post-thrombotic syndrome within two years, which involves chronic limb pain, swelling, heaviness, fatigue, and in extreme instances, limb ulceration.
Source venousnews.com
Duc Tin Clinic
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







