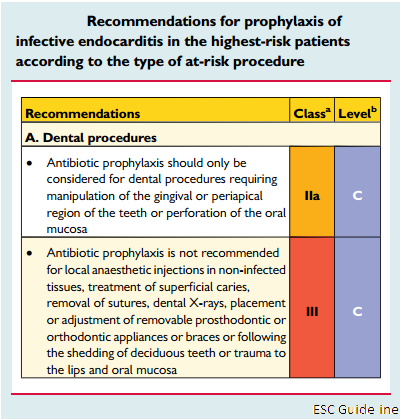
Antibiotic prophylaxis should only be considered for dental procedures requiring manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa including scaling and root canal procedures (Class IIa, Level C).
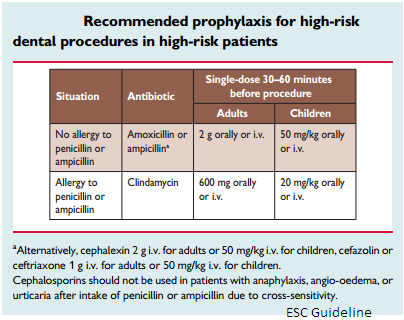
A. Prophylaxis for dental procedures in Infective Endocarditis.
| Antibiotic prophylaxis should only be considered for dental procedures requiring manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa including scaling and root canal procedures (Class IIa, Level C). The use of dental implants raises concerns with regard to potential risk due to foreign material at the interface between the buccal cavity and blood. But very few data are available. And there is no evidence to contraindicate implants in all patients at risk. The indication should be discussed on a case-by-case basis. The main targets for antibiotic prophylaxis in these patients are oral streptococci. Fluoroquinolones and glycopeptides are not recommended due to their unclear efficacy and the potential induction of resistance. Cephalosporins should not be used in patients with anaphylaxis, angio-oedema or urticaria after intake of penicillin or ampicillin due to cross-sensitivity. |
 |
|
B. Prophylaxis for non-dental procedures in Infective Endocarditis
In patients undergoing implantation of a prosthetic valve, any type of prosthetic graft or pacemakers, perioperative antibiotic prophylaxis should be considered due to the increased risk and adverse outcome of an infection. Prophylaxis should be started immediately before the procedure, repeated if the procedure is prolonged and terminated 48 h afterwards. The most frequent microorganisms underlying early (1 year after surgery) prosthetic valve infections are coagulase-negative staphylococci (CoNS) and Staphylococcus aureus. A randomized trial has shown the efficacy of 1 g intravenous (i.v.) cefazolin on the prevention of local and systemic infections before pacemaker implantation. Systematic local treatment without screening is not recommended. It is strongly recommended that potential sources of dental sepsis should be eliminated at least 2 weeks before implantation of a prosthetic valve or other intracardiac or intravascular foreign material, unless the latter procedure is urgent.
There is no compelling evidence that bacteraemia resulting from respiratory tract procedures, gastrointestinal or genitourinary procedures, including vaginal and caesarean delivery, or dermatological or musculoskeletal procedures causes IE.
Antibiotic therapy is only needed when invasive procedures are performed in the context of infection. And the patient at the highest risk for IE
(1) Patients with any prosthetic valve, including a transcatheter valve, or those in whom any prosthetic material was used for cardiac valve repair.
(2) Patients with a previous episode of IE.
(3) Patients with Congenital Heart Desease (CHD):
(a) Any type of cyanotic CHD.
(b) Any type of CHD repaired with a prosthetic material, whether placed surgically or by percutaneous techniques, up to 6 months after the procedure or lifelong if residual shunt or valvular regurgitation remains.
ESC Guideline
Duc Tin clinic
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389