The Path to Clinical Practice
Abstract.
Gene therapy, aimed at the correction of key pathologies being out of reach for conventional drugs, bears the potential to alter the treatment of cardiovascular diseases radically and thereby of heart failure.
Heart failure gene therapy refers to a therapeutic system of targeted drug delivery to the heart that uses formulations of DNA and RNA, whose products determine the therapeutic classification through their biological actions. Among resident cardiac cells, cardiomyocytes have been the therapeutic target of numerous attempts to regenerate systolic and diastolic performance, to reverse remodeling and restore electric stability and metabolism. Although the concept to intervene directly within the genetic and molecular foundation of cardiac cells is simple and elegant, the path to clinical reality has been arduous because of the challenge on delivery technologies and vectors, expression regulation, and complex mechanisms of action of therapeutic gene products. Nonetheless, since the first demonstration of in vivo gene transfer into myocardium, there have been a series of advancements that have driven the evolution of heart failure gene therapy from an experimental tool to the threshold of becoming a viable clinical option. The objective of this review is to discuss the current state of the art in the field and point out inevitable innovations on which the future evolution of heart failure gene therapy into an effective and safe clinical treatment relies.
Introduction
Gene-based therapies are gaining momentum from the incremental number of forthcoming clinical trials in human disease. With the recent approval of Glybera by the European Medical Agency (EMEA) as the first gene-based treatment of defective lipoproteinlipase activity, human gene therapy has become a clinical reality. The treatment introduces a normal lipoproteinlipase gene packaged in a delivery vector derived from adeno-associated virus (AAV), serotype 1 (AAV1), which conveniently has a natural propensity toward muscle cells, the tissue mainly contributing to healthy lipoproteinlipase protein production. Glybera is administered via a 1-time series of small intramuscular injections.
In human end-stage heart failure (HF), the Calcium Up-regulation by Percutaneous administration of gene therapy In cardiac Diseases (CUPID) trial (NCT00454818) pursues therapeutic levels of the calcium (Ca)2+-handling enzyme sarcoplasmic reticulum Ca2+ ATPase (SERCA2a).The second HF gene therapy phase I/II trial (Ad5.hAC6 Gene Transfer for Congestive Heart Failure; NCT00787059) seeking Food and Drug Administration (FDA)–granted investigational drug status uses human adenylyl cyclase 6 (AC6) as therapeutic target. If positively evaluated in forthcoming phase III studies, this novel therapeutics may open a new chapter in HF therapy. To date, these trials epitomize the quest for novel molecular-targeted HF treatments that could improve conventional clinical regimes, which cannot target underlying molecular defects of failing cardiomyocytes.
HF gene therapy refers to a therapeutic system that uses cardiac-directed delivery technology and viral vectors for biological drug administration to the diseased heart (Figure 1). Formulated DNA or RNA is used to intervene directly within the genetic and molecular foundation of diseased cardiomyocytes with the ultimate aim to correct key molecular defects being out of reach for conventional drugs. Using the cells own transcriptional and translational machinery, the classification (ie, inotropic) is determined by the biology of the DNA/RNA product. Since the first reports of in vivo gene transfer into the myocardium, there has been a series of advancements that have driven the evolution of an experimental tool to the threshold of becoming clinical reality.
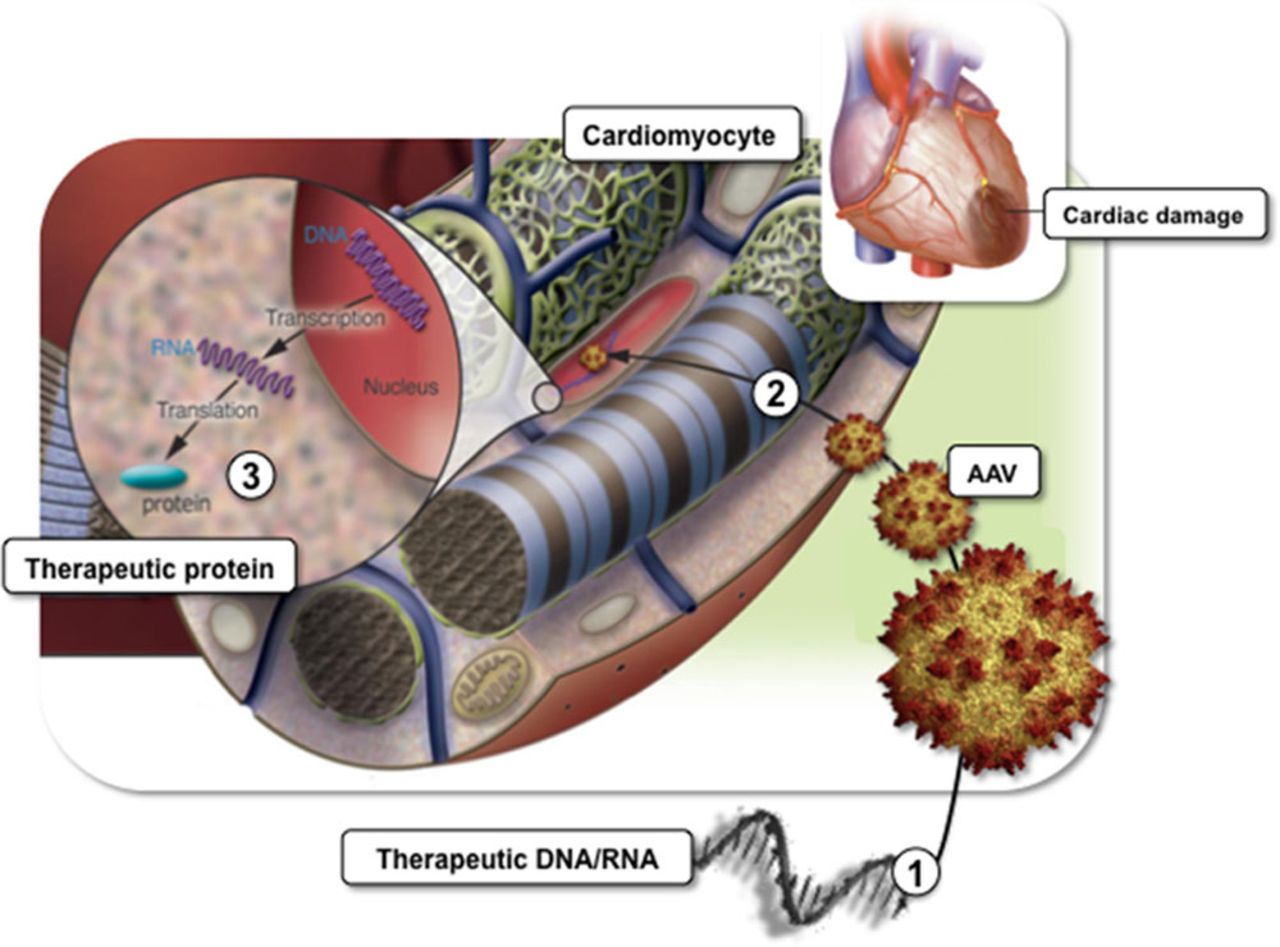
Figure 1.
Heart failure (HF) gene therapy concept. HF gene therapy uses viral vectors such as adeno-associated vectors (AAV; 1) to deliver therapeutic DNA and RNA to nuclei of dysfunctional cardiomyocytes and (2) to intervene directly within the genetic and molecular foundation of the cells. Myocardial infarction is shown as a common origin for HF development. Ultimate aim is targeted correction of key molecular defects being out of reach for conventional drugs using the cells own transcriptional and translational machinery (3). Adapted with permission from Davis et al (Physiol Rev. 2008;88:1567–651). Authorization for this adaptation has been obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Focusing on HF gene therapies that directly restore systolic cardiomyocyte performance, this review discusses state-of-the-art concepts and developments with respect to vectors, delivery technology, expression systems, and translation of targets into effective therapies. With respect to cardioprotective, paracrine, lusitropic, and angiogenic target biology, we refer to previously published excellent reviews. This article further highlights current needs to develop pharmacokinetic (PK) and pharmacodynamic (PD) models so that HF gene therapy can unleash its full potential as an effective and safe clinical treatment. It finally introduces the National Institutes of Health (NIH) Gene Therapy Resource Program (GTRP; http://www.gtrp.org) as catalyst for clinical translation and advocates the integration of systems biology to HF gene therapy.
Source ahajournal.org
DUC TIN surgical clinic
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







