Among different nonviral and viral vector systems developed for tissue and organ gene delivery, recombinant AAVs (rAAV) have emerged as the most valuable gene transfer agents available today (Figure 2). Because of their efficiency and safety in transducing both dividing and nondividing cells, rAAV vectors are used in >99 clinical trials worldwide (http://www.abedia.com/wiley/vectors.php).
Promising results have been obtained from phase I/II trials, including Leber’s congenital amaurosis, hemophilia B, Parkinson disease, cystic fibrosis, and, most recently, chronic HF. AAV1 is the viral vector used by the first EMEA-approved gene therapy targeting a rare human disease caused by defective lipoproteinlipase.
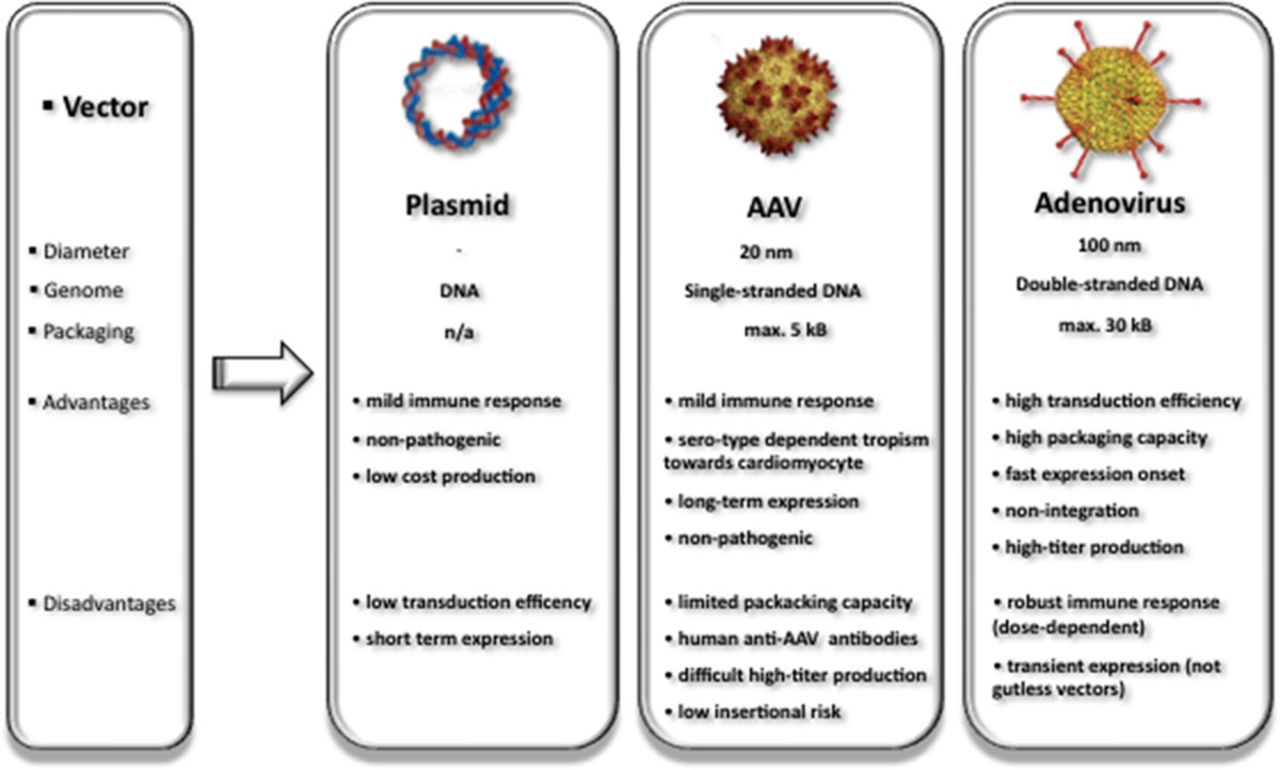
Figure 2.
Vectors commonly used in cardiovascular gene transfer and specific characteristics. To date, naked plasmid DNA, adeno-associated viruses (AAV), and adenovirus are used in 18.2%, 5.2%, and 23% of 1.902 registered gene therapy clinical trials (seewww.abedia.com/wiley/vectors.ph for continous update).
Wild-type AAV was first discovered as a 20-nm, icosahedral contaminant in adenovirus (Ad) preparations and belongs to the family of Parvoviridae. It is a nonpathogenic, nonenveloped DNA virus containing a linear single-stranded genome of 4.6 to 4.9 kb that requires coinfection with a helper virus for viral replication. Its genome consists of 2 open reading frames flanked by 145 base pair inverted terminal repeats. The genes encode alternatively spliced capsid (cap) proteins and multifunctional replication (rep) proteins (Figure 3A). To generate rAAV for gene delivery, a plasmid devoid of cap and rep genes is designed containing the control region and complementary DNA of interest flanked by wild-type AAV inverted terminal repeats. The only cis-elements apparently required for rAAV vectors are the inverted terminal repeats that flank the recombinant viral genome and aid in episomal concatamer formation in the nucleus after the single-stranded vector DNA is converted by host DNA polymerase into double-stranded DNA.
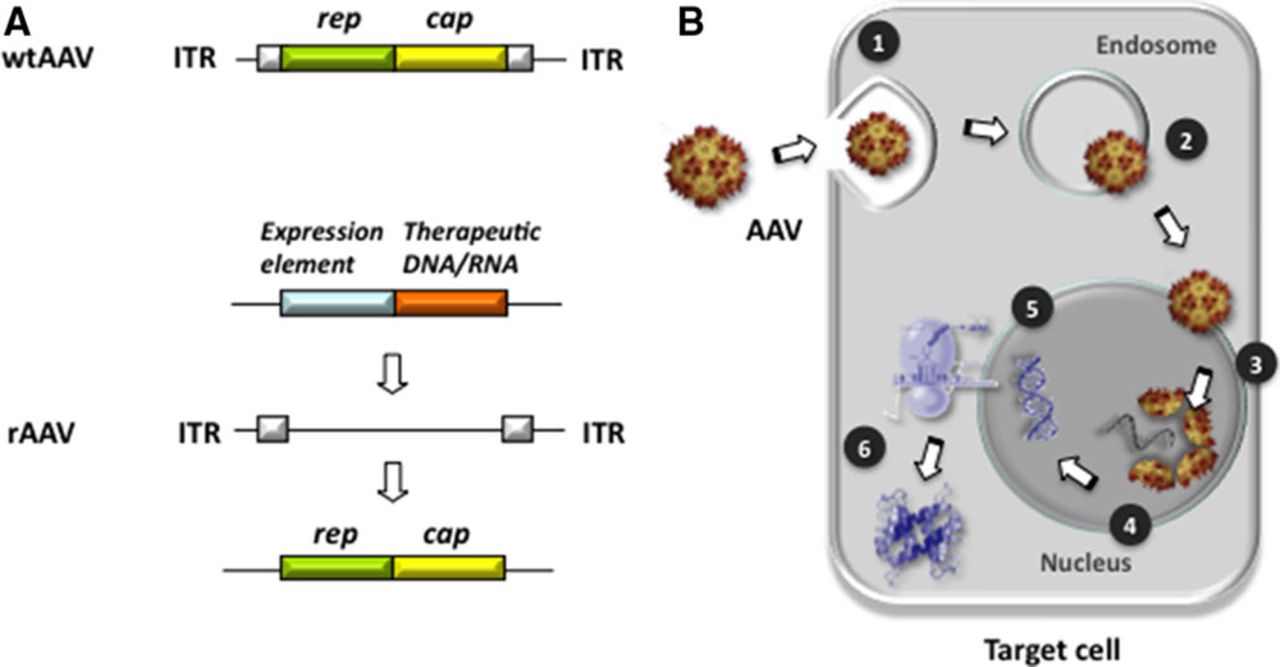
Figure 3.
Adeno-associated virus (AAV) genomic elements and nuclear entry. A, Structure and regulatory elements of wild-type AAV (wtAAV) and recombinant (rAAV) AAV. The wtAAV genome consists of replication (rep; green), capsid (cap; yellow), and internal repeat (ITR, white) elements. Exchange of rep/cap elements against therapeutic DNA/RNA (ie, expression regulation of DNA/RNA) the rAAV backbone with preserved ITRs. B, AAVs enter target cells via receptor binding entailing endocytosis (1). Once inside the cell, endosomal AAV escapes into the cytoplasm (2) and enters the nucleus via nuclear pore complexes (3). Nuclear import and capsid disassembly (4) are followed by release of AAV single-strand DNA payload and double-strand synthesis (5), which entails synthesis of the therapeutic protein/peptide (6).
The size of promoter elements and transgene is ideally between 4.1 and 4.9 kb for efficient packaging. The involved lack of expressing viral genes most likely contributes to the favorable low immunogenicity of rAAV when compared with other vectors. In contrast to the ability of wild-type AAV to integrate specifically into the host chromosome 19q13.4, rAAV serotypes used for cardiac gene delivery applications have been shown to persist largely as an episome for long periods of time after transduction. This accounts for >24 months of reported long-term cardiac expression duration, which renders AAV highly suitable for chronic HF therapy. Figure 3B illustrates key steps of cellular entry of AAV and its nuclear import in target cells.
Inherent cardiotropism, that is, supposedly preferential cardiomyocyte transduction of distinct rAAV serotypes such as rAAV9 in rodent models, adds to the attractive profile of the vector as current viral vehicle of choice irrespective of the in vivo delivery mode. Systemic application of rAAV9, for example, in rodents transduced both the heart and the extracardiac tissue (see the following section for details), but usage of a cardiac-directed promoter sequence restricted long-term expression of the encoded protein to the heart. Thus, the term cardiotropic for these vectors might somehow be misleading because it suggests cardiac restriction, which does not exist so far. Nonetheless, different options are currently being explored to develop rAAV mutants with optimal cardiotropism, cardiac specificity being the ultimate goal. One such possibility is transductional targeting, meaning directed capsid engineering and subsequent creation of mutant rAAV libraries. This can be achieved by random ligation of DNA fragments derived from different rAAV serotypes each encoding a different domain of the capsid protein; a technique also referred to as capsid DNA shuffling. Another method, directed capsid evolution, starts with the insertion of engineered peptide sequences into the AAV capsid thereby focusing on the major capsid protein VP3, which mostly accounts for receptor binding of AAV serotype to their target cells.
Both methods select the most promising cardiotrope rAAV variants by their either in vivo or in vitro transduction efficiency, and the corresponding capsid DNA is identified and sequenced by polymerase chain reaction amplification. In light of well-known differences of AAV serotypes with regard to species, optimization is mostly limited to the in vivo model used and cannot be extrapolated to other species, for example, from mouse to men. Repeated in vitro selection, on the other hand, is expected to deliver mutant rAAVs with optimal tropism toward isolated cardiomyocytes. Such selection neither predicts nor indicates in vivo cardiotropism, so that the ability of rAAV variants to overcome the endothelial barrier and interstitial space efficiently, as well as escape neutralizing antibodies, must be tested in vivo. Hence, transductional targeting is promising but of limited relevance when it comes to development of clinically applicable rAAVs and their cardiac specificity in humans.
Previously developed replication-deficient Ads (rAds) that also transfect both dividing and nondividing cardiac cells are still predominantly used in current clinical gene therapy trials. To date 437 clinical trials were registered using rAds (http://www.abedia.com/wiley/vectors.php). The most prominent example in cardiac gene therapy is the Ad5.hAC6 Gene Transfer for Congestive Heart Failure trial. Because antegrade coronary delivery was chosen as clinical delivery mode, the investigators preferred rAd5 to rAAV because of its natural ability to cross the endothelial barrier efficiently. As a major advantage, rAds provide a packaging capacity of ≤30 kb, exceeding by far the AAV size. First- and second-generation rAds, nonetheless, bear the disadvantage of significant immunogenicity, which reflects in a transient expression because of immunogenic elimination. Because a rAd-triggered innate immune response resulted in a previous trial participant’s death, a potential immune reaction poses obstacle safety issue to the wide-spread use of rAds tipping the balance toward AAVs for clinical cardiac gene delivery.
Source ahajournals.org
DUC TIN SURGICAL CLINIC
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







