To date, no delivery system or AAV serotype with optimized cardiotropism can rule out vector leakage or transduction of noncardiac tissues, respectively.
An efficient way to restrict therapeutic transgene expression is the use of cardiac-selective promoters referred to a transcriptional targeting: As concluded from numerous studies, engineered expression cassettes combining minimal fragments of genes that are exclusively expressed in cardiomyocytes with short enhancer sequences derived, that is, from viral promoters have shown most promising results with varying transgenes both in small and in large animal disease models. One example is the ventricle-specific myosin light chain-2 (MLC-2v) promoter ligated to a cytomegalovirus (CMV) enhancer fragment driving cardiac-selective expression both of S100A1 and βARKct without detectable noncardiac tissue expression after retrograde intravenous cardiac delivery. Another selective promoter uses a fragment of the cardiac α-actin enhancer sequence attached to the elongation factor 1α to achieve cardiac-specific expression of the same transgenes. Importantly, after intracoronary and intravenous delivery, no unwarranted expression of S100A1 or βARKct was observed in noncardiac tissues.
Hence, transcriptional targeting further aids in decreasing the risks of systemic or side effects, rendering these systems suitable for human use. It is, however, important to note that in analogy to conventional drugs, the therapeutic window of a therapeutic gene product can vary widely. As seen with many transgenes, there is a considerable amount of empiricism in the choice of the promoter. Before its administration, a constitutively active one typically yields unpredictable expression levels that could lie either inside or outside the therapeutic window accounting for inefficiency and toxicity. Just as efficacy of conventional drugs relies on accurate understanding of dose–response relationship and PK/PD data in view of therapeutic window and fluctuations of disease severity, so does effectiveness of therapeutic gene delivery, thus demanding adjustable expression level and time course of the gene product. This type of control may be achieved by incorporation of engineered gene switches (Figure 5). Among the various available gene-switch platforms, the insect-derived ligand-inducible, ecdysteroid receptor system, which is refractory to human endogenous steroids, typically shows low basal transgene expression, broad dose–response gradation, and high inducibility. It usually outperforms other systems such as the tetracycline- or rapamycin-regulated systems that have been used in the context of AAV vectors.
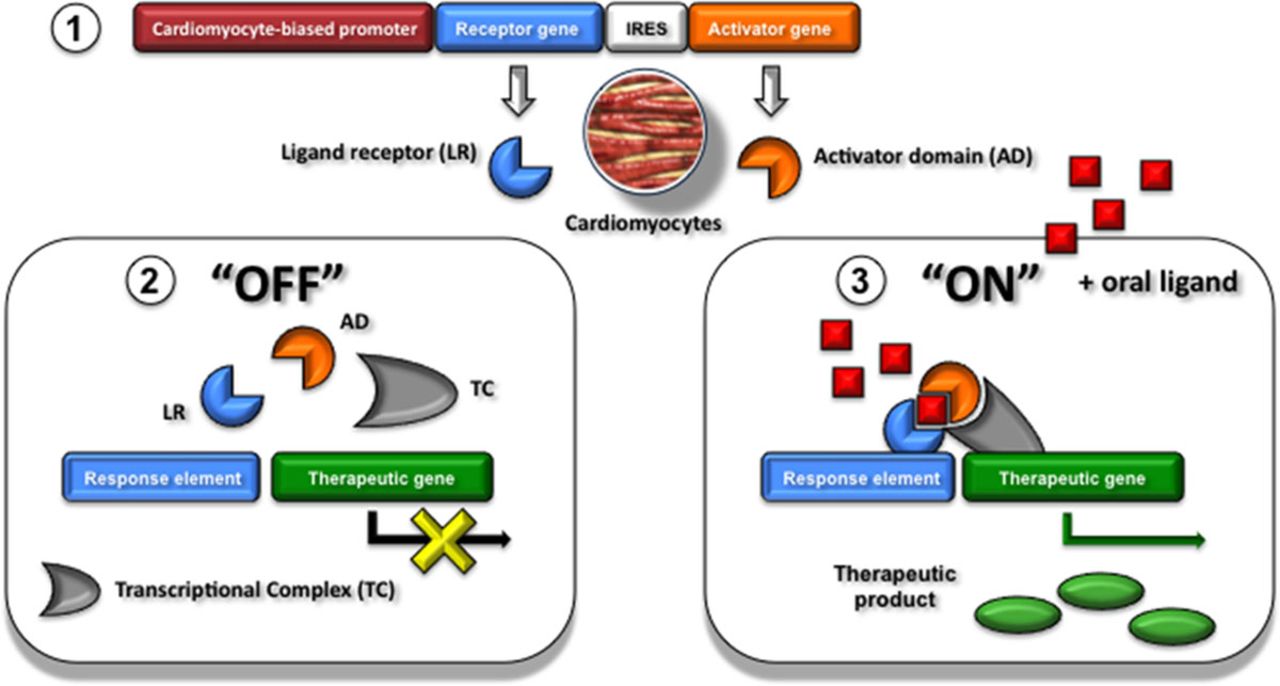
Figure 5.
Cardiac-biased controllable gene expression systems. Transcriptional control using a cardiomyocyte-biased controllable gene expression system (1). In the absence of the oral ligand (OFF-state), the cardiomyocyte specifically expressed ligand receptor (LR; blue) and activator domain (AD; orange) exist in an inactive conformation and transcription is kept off (2). Ingestion of the oral ligand leads to the ON State: in the presence of ligand (red), the 2 proteins (LR and AD) stably dimerize. The complex in an active conformation binds to the response element of the codelivered therapeutic gene and transcription is turned on. IRES indicates internal ribosomal entry site.
In addition, expression cassettes have been developed whose activity is governed by a disease condition. One such example is the hypoxia response element concatamer of the erythropoietin gene combined with a cardiac-specific promoter fragment that becomes active only under ischemic conditions. Other useful combinations currently under development comprise the use of shorter versions of the brain natriuretic peptide promoter to control therapeutic transgene expression in the heart’s response to mechanical stress. In turn, brain natriuretic peptide–driven therapeutic genes capable to improve contractile performance and thereby reducing ventricular wall stress could establish a therapeutic feedback loop that automatically adjusts the level of transgene expression to changes in ventricular wall tension. In light of the limited packaging capacity of AAV vectors, targeted and inducible systems, however, still come at the price of reducing the allowable size of the therapeutic transgene to ≈0.8 to 1 kb. Options are currently being explored to overcome the limited coding capacity. One such possibility is that the AAV inverted terminal repeats of 2 genomes can anneal head to tail to form concatamers, almost doubling the capacity of the vector.
For future clinical use, spatial and temporal control of the expression of the therapeutic gene product should, therefore, not just be considered as merely desirable but rather as an indispensable instrument to adjust the intracellular dosage to treat effectively while staying within the safety range. Such clinical implicitness eventually constitutes the necessity for development of alternative vector systems beyond current AAV technology providing sufficient capacity for targeted and regulated expression systems. As we look forward to the next generation of cardiac gene therapy studies, constitutively active, nontargeted expression is likely to lose ground in favor of more advanced technologies.
Source ahajournals.org
DUC TIN SURGICAL CLINIC
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







