It was anticipated that therapeutic targets identified and tested in animal models with critical features of HF in humans have a higher likelihood of translating to patients (Figure 6). Given distinct advantages and shortcomings of small and large animal HF models,
efficient preclinical translation rests on targeted use of model systems that best fit specific requirements of each developmental stage. Sound understanding of the molecular mode of action of a target is a conditio sine qua non before any translational attempts to validate a predicted therapeutic potential. All targets originating from reverse (bedside to bench and back) or forward (bench to bedside) translation require proof of their therapeutic potency in preclinical disease models. Despite differences to humans with respect to cardiac physiology (ie, higher heart rate, larger SERCA2a/sodium/calcium exchanger (Na+/Ca2+ exchanger) ratio, and difference in energy homeostasis), anatomy and molecular composition (ie, higher capillary density, smaller hearts, and different sarcomeric proteins), rodents will remain the cornerstone for proof-of-concept studies allowing for efficient testing of phenotype and outcome.
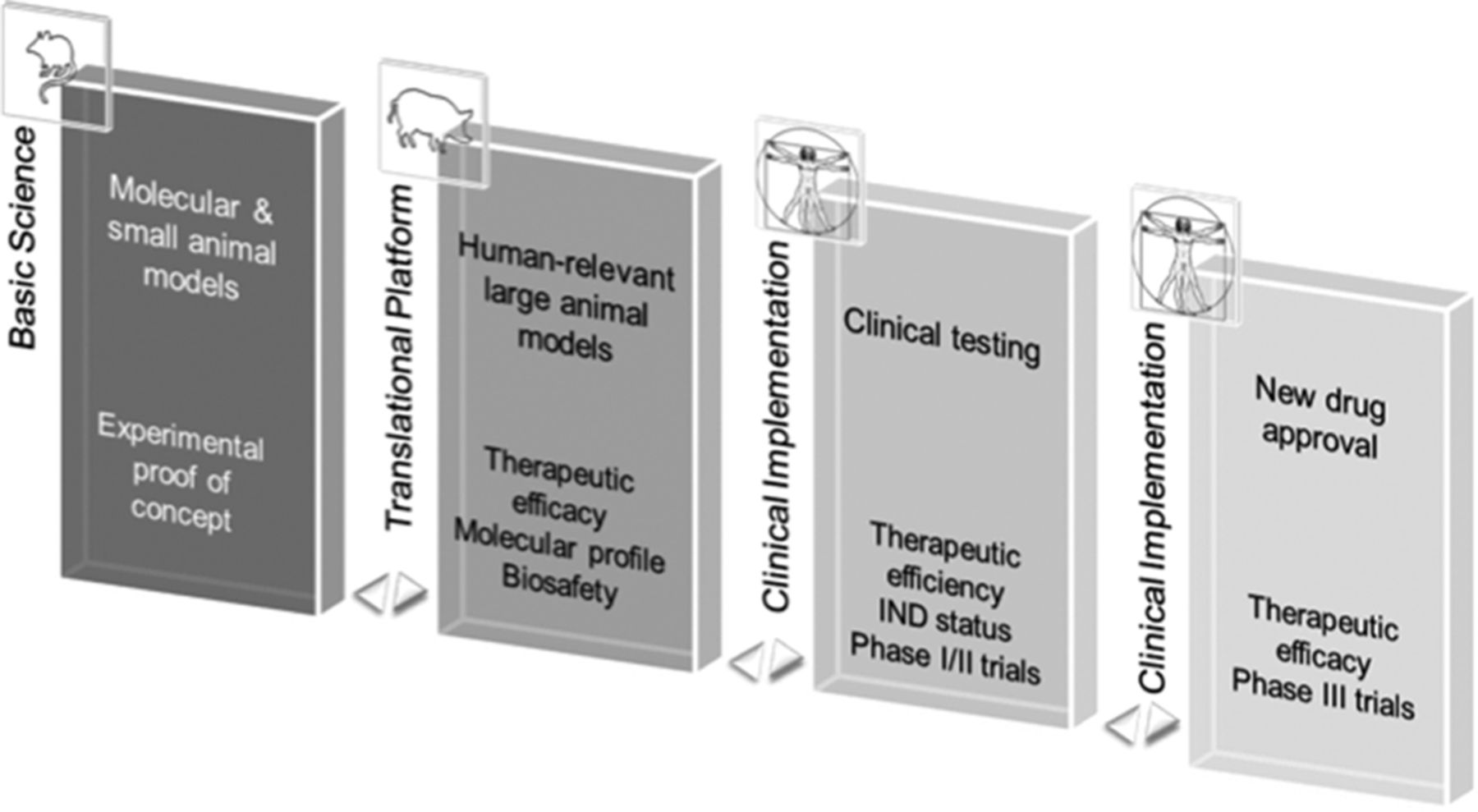
Figure 6.
Translational strategy for preclinical heart failure (HF) gene therapy development. Integrative pipeline for preclinical development of HF and other cardiovascular gene therapy. For successful clinical implementation, experimental therapeutic proof-of-concept and molecular profile are ideally determined in molecular and small animal models (Basic Science). Human-relevant large animal disease models are key for translation toward use in humans enabling development and testing of clinically applicable delivery technology and target efficiency and safety. Data gleaned from this stage are essential for investigational drug (IND) status application and transition to clinical safety and exploratory dosing (phase I/II) trials in humans. Efficacy assessment (phase III) concludes successful clinical translation. Adapted with permission from Ritterhoff and Most. Authorization for this adaptation has been obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Moreover, cost-effectiveness, smaller dosages of vectors, and various available cardiac disease models (ie, permanent myocardial infarction [MI], ischemia/reperfusion, transaortic constriction) together with advanced echocardiography, invasive hemodynamic assessment, and MRI technology enable efficient analyses. In addition, numerous high-throughput tools have been developed toward rodent models enabling large-scale collection of molecular data and holistic mechanism of action (see PK and PD of Cardiac Gene Therapeutics section for details) associated with the phenotype. Taken together, ease of therapeutic manipulation and insight into molecular mechanisms in greater animal numbers with more substantial statistical power makes them an indispensable part of a standardized translational platform concept to guide development of HF gene therapeutics.
Human failing and normal cardiomyocytes are other highly valuable models to assess the therapeutic efficacy of a target. Although difficult isolation procedure and scarcity of explanted human hearts pose a challenge for sufficient cell numbers and quality, the in vitro model remains the only option to test molecular therapeutics in diseased human myocardium. Importantly, human cardiomyocytes provide features not present in rodent cardiomyocytes, such as force-frequency response and β-adrenergic receptor (β-AR) compositions, strengthening the predictive value of a positively evaluated therapeutic target. With a sound understanding of its therapeutic mode of action and efficiency in small animal models, a target is principally amenable for translation to large animal HF models.
Because of closer approximation of human cardiovascular physiology and pathology, large animal models are the critical step in the translation of advanced drug, cell, and gene therapeutics to clinical practice. They pose practical questions as to scalability of a particular therapeutic, dosage, and delivery method. Studies in large animal models usually require 10- to 100-fold greater amounts of therapeutic viral particles and provide preclinical data with respect to feasibility, efficacy, and biological safety for novel therapies before FDA-approved commencement of clinical trials. Pigs have emerged as the species of choice in the field of cardiac gene therapy for several reasons.56. Although pig genome mapping has successfully spurred the evolution of high-throughput omics technologies, a broader use of the model is still hampered by a lack in basic molecular biology tools (ie, antibodies approved or designed for the use in pigs). In addition, new research facilities often lack the considerable space and resources demanded by large animal facilities, and to perform preclinical studies requires a multidisciplinary team of scientists and clinicians. Ideally, research and clinical facilities provide the teams at this stage with access to state-of-the-art clinical and molecular diagnostic and analytic tools, ranging from hemodynamic (ie, MRI, pressure–volume-loop measurements, and echocardiography), electrophysiological (ie, 3-dimensional cardiac mapping, telemetric ECG recordings) to metabolic (ie, positron-emission tomography [PET]- computed tomography [CT], single photon emission computed tomography, and metabolic assay platform) and systems biology tools. Assembling such a powerful toolkit is necessary to advance the informative value of the costly model beyond integrative cardiovascular physiology.
The NIH GTRP (http://www.gtrp.org) provides a framework to promote clinical translation of advanced HF gene therapies to enter the application process for an investigational drug status. As a unique catalyst of gene-based therapies, the GTRP provides professional support in the area of required good manufacturing/laboratory practice testing of therapies, pharmacology–toxicology studies, good manufacturing practice–grade vector production, and regulatory support in accompanying interactions with the FDA. The next steps include design, planning, and cost calculation of forthcoming clinical trials, selection of competent good manufacturing practice/good laboratory practice certified contract-research companies, and eventually development of a business model to seek sufficient funding by a financial or industrial partner.
Source ahajournals.org
DUC TIN Surgical Clinic
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







