AAAs arise as a result of a failure of the major structural proteins of the aorta (elastin and collagen). The inciting factors are not known, but a genetic predisposition clearly exists.
Although aneurysms represent a dilatation in all layers of the vessel wall, AAAs develop after degeneration of the media. The degeneration ultimately may lead to widening of the vessel lumen and loss of structural integrity.
After age 50 years, the normal diameter of the infrarenal aorta is 1.5 cm in women and 1.7 cm in men. An infrarenal aorta that is 3 cm or more in diameter is considered an AAA, even if asymptomatic. Approximately 90% of AAAs are infrarenal.
A multidisciplinary research program supported by the US National Heart, Lung, and Blood Institute identified the following as mechanisms important in the development of AAA:
-Proteolytic degradation of aortic wall connective tissue
-Inflammation and immune responses
-Biomechanical wall stress
-Molecular genetics
Similarly, surgical specimens of AAA reveal the following (see the image below):
-Inflammation, with infiltration by lymphocytes and macrophages
-Thinning of the media
-Marked loss of elastin
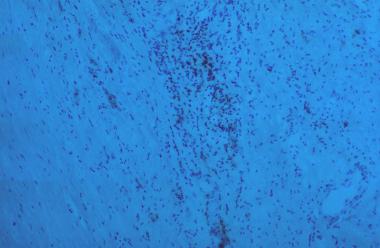
Inflammation, thinning of media, and marked loss of elastin.
Elastin
Elastin is the principal load-bearing element in the aorta. The aortic wall contains smooth muscle, elastin, and collagen arranged in concentric layers in order to withstand arterial pressure. The number of medial elastin layers is markedly reduced from the proximal thoracic aorta to the infrarenal aorta, with medial thinning and intimal thickening.
Elastin fragmentation and degeneration are observed in aneurysm walls. The decrease in content coupled with the histologic changes of this matrix protein in aneurysms may explain the propensity for aneurysm formation in the infrarenal aorta.
Proteolysis, metalloproteinases, and inflammation
In AAA, the aortic media appears to degrade by way of a proteolytic process. This implies an increase in the concentration of proteolytic enzymes relative to the concentration of their inhibitors in the abdominal aorta as the individual ages.
Some research has focused on the role of the metalloproteinases, a group of zinc-dependent enzymes responsible for tissue remodeling. Reports have documented increased expression and activity of matrix metalloproteinases (MMPs) in people with AAAs. MMPs and other proteases have been shown to be secreted into the extracellular matrix of AAAs by macrophages and aortic smooth-muscle cells.
MMPs and their inhibitors are present in normal aortic tissue and are responsible for vessel-wall remodeling. Aneurysmal tissue tends to demonstrate increased MMP activity and decreased inhibitor activity, which favor the degradation of elastin and collagen. The mechanism that tips the balance in favor of degradation of elastin and collagen in the aortic wall of AAAs by MMPs and other proteases is not yet known.
Upon histologic examination, AAAs demonstrate a chronic adventitial and medial inflammatory infiltrate. Infiltration of AAAs with lymphocytes and macrophages may trigger protease activation via various cytokines (eg, interleukin [IL]–1, IL-6, IL-8, and tumor necrosis factor [TNF]–α).
Immunoreactive proteins are found more conspicuously in the abdominal aorta, and this may contribute to the increased frequency of aneurysms in this location. Further study has defined a matrix protein that is immunoreactive with immunoglobulin G in the aneurysm wall. This autoantigen appears to be a collagen-associated microfibril. Certain infectious agents (eg, Chlamydia pneumoniae and Treponema pallidum) have been associated with the development of this protein; however, no direct cause-and-effect relation has been demonstrated.
Insights from molecular genetics
Through gene microarray analysis, various genes involved in extracellular matrix degradation, inflammation, and other processes observed in AAA formation have been shown to be upregulated, whereas others that may serve to prevent this occurrence are down-regulated. The combination of proteolytic degradation of aortic-wall connective tissue, inflammation and immune responses, biomechanical wall stress, and molecular genetics represents a dynamic process that leads to aneurysmal deterioration of aortic tissue.
Atherosclerosis
Most AAAs occur in individuals with advanced atherosclerosis. Atherosclerosis may induce AAA formation by causing mechanical weakening of the aortic wall with loss of elastic recoil, along with degenerative ischemic changes, through obstruction of the vasa vasorum.
Many patients with advanced atherosclerosis do not develop AAA, and some patients having no evidence of atherosclerosis do. The observed association between atherosclerosis and AAA probably is not causative; however, atherosclerosis may represent a nonspecific secondary response to vessel-wall injury that is induced by multiple factors.
Source emedicine.com
Duc Tin Surgical Clinic
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







