A meta-analysis comparing percutaneous coronary intervention (PCI) using a drug-eluting stent (DES) to coronary artery bypass graft (CABG) surgery in patients with left main coronary artery disease has concluded there is no statistically significant difference between the two strategies in terms of mortality at five years.
Nonagenarians should not be refused transcatheter aortic valve implantation (TAVI) based solely upon their age, the findings of an analysis presented at the European Society of Cardio-Thoracic Surgery annual meeting (EACTS 2021; 13–16 October; Barcelona, Spain and virtual) suggest. This was the conclusion of Roxanne St-Louis (Quebec Heart and Lung University Institute, Quebec, Canada) who detailed a retrospective review of the survival benefit among nonagenarians undergoing TAVI.
An updated risk score to predict possible contrast-associated acute kidney injury (CA-AKI) among patients undergoing percutaneous coronary intervention (PCI) has been presented by Roxana Mehran (Icahn School of Medicine at Mount Sinai, New York, USA) at the American Heart Association’s Scientific Sessions 2021 (AHA 2021; 13–15 November; virtual).
Use of a novel, venous external support device (VEST, Vascular Graft Solutions) may lead to increased longevity and durability of saphenous vein grafting during coronary artery bypass graft (CABG) surgery, according to investigators in the VEST trial, results of which were present by John Puskas (Icahn School of Medicine at Mount Sinai, New York, USA) at the American Heart Association’s Scientific Sessions 2021 (AHA 2021; 13–15 November; virtual).
Coronary artery bypass graft sugery (CABG) between two and three days after ticagrelor cessation was found to be non-inferior in incurring severe or massive perioperative bleeding compared to waiting between five and seven days prior to the procedure, per current guidelines.
Caption Health and Ultromics have announced a strategic partnership to accelerate cardiovascular disease detection and treatment for more patients in more accessible care settings. Together, the companies will jointly offer the Caption AI software platform alongside Ultromics’ EchoGo deep ultrasound analytics.
CoreMedic has announced the publication of two-year follow-up results from the first in-human CHAGALL trial of its ChordArt mitral valve repair device in the treatment of mitral valve regurgitation (MR). Chordal replacement with ChordArtTM is reported to be safe, effective, and durable at two years follow up.
Data from the BIOSOLVE-IV trial, presented at the Transcatheter Cardiovascular Therapeutics annual meeting (TCT 2021, 4–6 November, Orlando USA and virtual), demonstrate the superiority of the Magmaris (Biotronik) sirolimus-eluting bioresorbable magnesium scaffold with regard to probable or definite scaffold thrombosis compared to a historical control group of the Absorb (Abbott) bioresorbable scaffold.
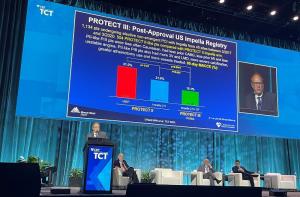
The final results of the PROTECT III and Restore EF prospective studies demonstrate improved outcomes for high-risk PCI patients with the use of the Impella (Abiomed) heart pump.
Percutaneous coronary intervention (PCI) guided by fractional flow reserve (FFR) did not meet non-inferiority for one-year adverse events compared to coronary artery bypass grafting (CABG) in patients with three-vessel coronary artery disease, primary results of the FAME 3 (Fractional flow reserve versus angiography for multivessel evaluation) trial have shown.
ReValve Solutions has announced that it has successfully completed the first-in-human use of its transcatheter Palmetto system for mitral valve replacement.
Results from two studies of the Impella heart pump device will be presented at the Transcatheter Cardiovascular Therapeutics annual meeting (TCT 2021, 4–6 November, Orlando USA and virtual).

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389