Endologix has announced the first commercial implant and the US commercial release of its recently US Food and Drug Administration (FDA)-approved Alto endograft for the treatment of abdominal aortic aneurysms (AAA).
Routine ultrasound-guided percutaneous transluminal angioplasty (PTA) is a feasible treatment for native arteriovenous fistula (AVF) dysfunction, according to a recent study by Antonio Granata (“Cannizzaro” Hospital, Catania, Italy). The findings were published online on 25 July in the Journal of Vascular Access (JVA).
Artio Medical today announced it has acquired Flow Forward Medical, a medical device company developing methods for establishing and maintaining vascular access sites.
A German study of recently-recovered COVID-19 patients examined using magnetic resonance imaging (MRI) revealed cardiac involvement in 78% of patients, and ongoing myocardial inflammation in 60%. Published online in JAMA: Cardiology, the study’s findings indicate the need for ongoing investigation of the long-term cardiovascular consequences of COVID-19, according to lead author Valentina Puntmann (University Hospital Frankfurt, Frankfurt, Germany) and colleagues.
One-year outcomes of mitral valve-in-valve replacement using the Sapien 3 (Edwards Lifesciences) transcatheter heart valve have been published in JAMA: Cardiology, reporting low all-cause mortality at 30 days (5.4%) and one-year (16.7%), and a 96.8% procedural success rate.
Keystone Heart has announced completion of the first worldwide commercial case using the TriGUARD 3 cerebral embolic protection (CEP) device. Pieter Stella, assistant professor, Medical Department of Cardiology, at UMC Utrecht, Utrecht, the Netherlands, performed the transcatheter aortic valve implantation (TAVI) procedure using the TriGUARD 3 device, which is designed to minimise the risk of cerebral damage during transcatheter heart procedures.
Use of non-steroidal anti-inflammatory drugs (NSAIDs) to treat first-time heart attack patients significantly increased the risk for cardiovascular and bleeding events post-heart attack in a nationwide Korean study, published in the Journal of the American College of Cardiology.
The US Food and Drug Administration (FDA) has approved one-way digital data streaming during patient support from Abiomed’s Automated Impella Controller (AIC), the external console used with Impella heart pumps.
Genetic testing and counselling for inherited cardiovascular diseases may help patients and their families make informed decisions about managing their heart health, according to Genetic Testing for Inherited Cardiovascular Diseases, a scientific statement from the American Heart Association (AHA), published today in the Association’s journal Circulation: Genomic and Precision Medicine.
Boston Scientific has received US Food and Drug Administration (FDA) approval for the Watchman FLX left atrial appendage closure (LAAC) device. The device is indicated to reduce the risk of stroke in patients with non-valvular atrial fibrillation (NVAF) who need an alternative to oral anticoagulation therapy by permanently closing off the left atrial appendage—the area of the heart where stroke-causing blood clots commonly form in NVAF.
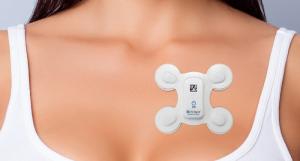
LifeSignals Group has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for the LifeSignals ECG Remote Monitoring Patch platform.
A rise in the number of out-of-hospital cardiac arrests (OHCAs) correlated with a fall in the number of acute coronary syndrome hospitalisations during the COVID-19 surge in the New York metropolitan area, according to a research letter published in the Journal of the American College of Cardiology (JACC

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389