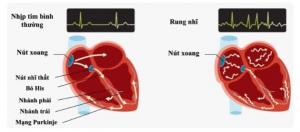
Acutus Medical has announced CE mark approval and the European launch of its integrated family of transseptal crossing products, designed to deliver safe and efficient access to the left atrium. Coupled with their previously received FDA clearance, Acutus now has regulatory approval to gain access to key global geographies in the large and growing market for transseptal crossing products.
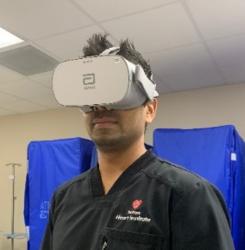
Abbott has announced the global release of its first virtual reality-based training programme designed to change training for interventional cardiologists in the use of optical coherence tomography (OCT) imaging technology.
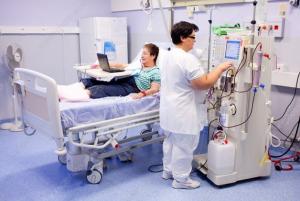
Hypercoagulability in COVID-19 patients leads to an increase in the malfunction rate of temporary haemodialysis catheters—but heparin locking of the catheters is linked to decreased malfunction rates. Those are among the main findings of a study recently published in the Journal of Vascular Surgery.
Terumo Aortic has announced the midterm results from the RelayPlus thoracic stent graft system post-approval study, revealing low operative mortality and morbidity—supporting its use as a “safe and effective” thoracic aortic aneurysm treatment.
There is no evidence of a benefit from additional paclitaxel-coated balloon use compared to standard balloon angioplasty alone in the context of preserving arteriovenous (AV) fistula patency for haemodialysis, according to as-yet unpublished data from the multicentre, randomised controlled PAVE (Paclitaxel-coated balloons and angioplasty of AV fistulas) trial, presented at the 2020 annual scientific meeting of the British Society of Interventional Radiology (BSIR; 1–3 December, online). Michael Robson (Guy’s and St Thomas’ Hospital and King’s College London, London, UK) informed livestream attendees that there was no indication of an early treatment effect, and that all primary and secondary outcome measures of their trial demonstrated this same conclusion. Robson reported no disclosures relating to the PAVE trial, but noted that BD provided the balloons used. BD played no other role in the investigation, he reported.
B Braun has revealed that 12-month results from the LOCOMOTIVE EXTENDED study of the company’s Multi-Loc multiple stent delivery system have been published in Vasa: European Journal of Medicine. The study authors, Klaus Amendt (Diakonissenkrankenhaus Mannheim, Mannheim, Germany) and colleagues conclude that the Multi-Loc “provides promising results concerning target lesions revascularisation and primary patency at 12 months”.
LivaNova has entered into an agreement with investment firm Gyrus Captial whereby entities funded and controlled by Gyrus will acquire the LivaNova heart valve (HV) business.
Baylis Medical has announced the publication of data demonstrating procedural efficiency for MitraClip transcatheter mitral valve repair (TMVR) with use of its VersaCross RF Transseptal device, reporting guide delivery from femoral access in under 7.5 minutes.
Gore has announced encouraging clinical results from its early feasibility study evaluating the safety and performance of its pulmonary valved conduit, an investigational device. Six-month data for 16 patients enrolled across three sites in the USA were presented in a moderated poster session during the American Heart Association virtual scientific sessions (AHA 2020, 13 – 17 November, virtual).
Emerging data supports transcatheter techniques to treat primary mitral regurgitation, according to KK Yeo (National Heart Center, Singapore) who presented at PCR Valves 2020 (22–24 November, virtual) discussing the latest developments in edge-to-edge repair for primary mitral regurgitation.
MedAlliance has announced completion of patient enrolment in the ISABELLA clinical trial with the Selution SLR 018 drug-eluting balloon (DEB) for the treatment of dysfunctional arteriovenous (AV) fistulas in end-stage renal failure patients undergoing haemodialysis.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389