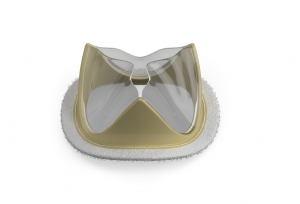
Fist Assist Devices has announced that it recently received Breakthrough Device designation from the US Food and Drug Administration (FDA) for its Fist Assist Model FA-1 device.
Research indicates that healthcare professionals perform better than risk prediction tools at predicting 30-day mortality, morbidity, and revision risk in patients following major lower limb amputation. The PERCEIVE study is led by chief investigator David Bosanquet (Royal Gwent Hospital, Newport, UK), funded by Health and Care Research Wales’ Research for Patient and Public Benefit scheme, and delivered by the Centre for Trials Research (Cardiff University) and the Vascular and Endovascular Research Network (VERN). Presenting early data from the PERCEIVE study during the Sol Cohen Prize Session at the UK Vascular Societies’ Annual Scientific Meeting (VSASM 2021; 1–3 December, Manchester, UK), Brenig Gwilym (Royal Gwent Hospital) stressed that determining the accuracy of healthcare professionals’ predictions is extremely important when validating risk prediction tools to put their performance into context.
Royal Philips has announced US Food and Drug Administration (FDA) de novo clearance for the Philips inferior vena cava (IVC) filter removal laser sheath—CavaClear—to remove an IVC filter when previous methods of removal have failed.
Royal Philips has announced US Food and Drug Administration (FDA) de novo clearance for the Philips inferior vena cava (IVC) filter removal laser sheath—CavaClear—to remove an IVC filter when previous methods of removal have failed.
Foldax has announced publication of the first clinical results for its Tria biopolymer heart valve, which the company describes as the first polymer valve to be implanted in humans.
Edwards Lifesciences has received approval from the US Food and Drug Administration (FDA) for the use of the Edwards Sapien 3 transcatheter valve with the Alterra adaptive prestent—Sapien 3 with Alterra—for patients with severe pulmonary regurgitation.
Doctors at St David’s Medical Center in Austin, USA recently implanted a new neurostimulator technology in patients to help treat advanced heart failure—and are among the first physicians in the USA to do so, according to a press release.
Many patients undergoing cardiac surgery may be able to safely and effectively control postoperative pain without opioids after hospital discharge, according to research published in The Annals of Thoracic Surgery.
Ultromics has announced the publication of a study assessing its EchoGo platform, powered by Artificial Intelligence (AI), which it says significantly improved the accuracy and confidence in coronary artery disease (CAD) detection from a stress echocardiogram.
Terumo Aortic has announced that the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has granted approval of the RelayProthoracic stent graft system for sale in Japan for the treatment of patients with thoracic aortic aneurysms (TAAs).
HeartFlow has announced it has submitted a 510(k) premarket application to the US Food and Drug Administration (FDA) to add advanced anatomic assessment and plaque evaluation to the HeartFlow FFRct Analysis.
Svelte Medical Systems has received US Food and Drug Administration (FDA) approval to commercialise the Slender IDS fixed-wire and Direct RX rapid-exchange drug-eluting stent (DES) systems for the treatment of coronary artery disease in the USA.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389