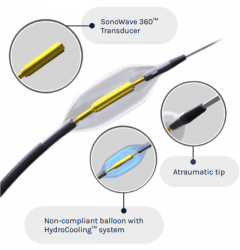
Corindus has been rebranded to Siemens Healthineers Endovascular Robotics and will sit as a dedicated business within the Advanced Therapies area of Siemens Healthineers, the company has announced.
A first-in-human trial using a purpose-built device to provide direct wire pacing without the need for a temporary venous pacemaker during transcatheter aortic valve implantation (TAVI) and complex percutaneous coronary intervention (PCI) has found that the device was safe and effective.
Otsuka Medical Devices and ReCor Medical, a subsidiary of Otsuka, announce the filing of the pre-market approval (PMA) application to the US Food and Drug Administration (FDA) for the Paradise ultrasound renal denervation system (uRDN) system in the treatment of uncontrolled hypertension.
Data presented at an innovation session at the 2022 Structural and Coronary Intervention Course (CSC, 16–18 November, Madrid, Spain) demonstrated an equivalent performance of the Xlimus (Cardionovum) drug-eluting stent (DES) in terms of optical coherence tomography (OCT)-derived endpoints than the Synergy (Boston Scientific) DES, including neointimal volume and neointimal volume weighted by length at six months.
Bivalirudin is a safer and more effective anticoagulant than heparin for treating patients with ST-segment elevation myocardial infarction (STEMI) who undergo primary percutaneous coronary intervention(PCI), and can lower the risk of death or major bleeding by 31%.
The American College of Cardiology (ACC) and American Heart Association (AHA) have jointly published new guidelines on the diagnosis and management of aortic disease.
Researchers are to investigate the safety and efficacy of performing left atrial appendage occlusion in patients at high risk for atrial fibrillation (AF) who are undergoing cardiac surgery to prevent future stroke.
CathWorks has announced approval of the fourth generation CathWorks FFRangio system by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA). The FFRangio system is also commercially available in the USA and Europe.
Terumo Aortic today announced that the US Centers for Medicare and Medicaid Services (CMS) has granted approval of a new technology add-on payment (NTAP) for Terumo Aortic’s Thoraflex Hybrid device under the inpatient prospective payment system (IPPS).
Cardionovum has announced the enrolment of the first patient in the international Hyper II clinical study designed to evaluate the safety and efficacy at 12 months of the hybrid treatment approach combining the Restore drug-coated balloon (DCB) with new a generation drug-eluting stent (DES) in long diffuse coronary artery disease (CAD).
Abbott has announced the release of late-breaking showing that its HeartMate 3 heart pump extends survival of advanced heart failure patients by at least five years.
The timing of dosing of antihypertensive medication—whether it is taken in the morning or in the evening—does not have a bearing on cardiovascular outcomes, randomised trial results presented during a hot line session at the European Society of Cardiology (ESC) annual congress (26–29 August, Barcelona, Spain) indicate.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389