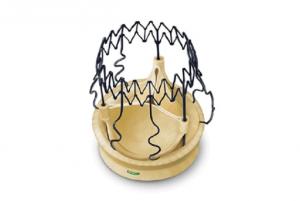
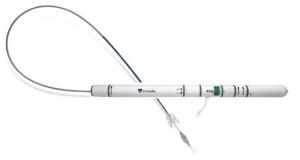
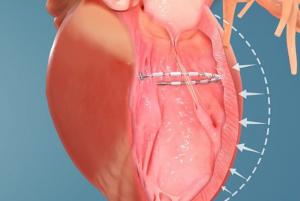
LivaNova has announced that the advanced Perceval Plus sutureless surgical aortic heart valve is now available for commercial release in Europe, having successfully completed a one-year limited launch with initial real-world clinical data gathering.
Edwards Lifesciences has announced approval from the US Food and Drug Administration (FDA) for the KONECT RESILIA aortic valved conduit (AVC), a ready-to-implant solution for bio-Bentall procedures, a surgery that involves replacement of a patient’s aortic valve, aortic root and the ascending aorta.
Venus Medtech has announced a collaboration to bring Pi-Cardia’s Leaflex technology to China.
MedAlliance has announced enrollment of the first patient in its study of Selution SLR 014” drug eluting balloon (DEB) for the treatment of in-stent restenosis. According to the company, this is the first DEB to be accepted by the US Food and Drug Administration (FDA) for its Breakthrough Programme. The Selution SLR (sustained limus release) is a novel sirolimus eluting balloon that provides a controlled sustained release of drug, similar to a drug-eluting stent (DES).
The European Association of Percutaneous Cardiovascular Interventions(EAPCI) has published a consensus document on ischaemia with non-obstructive coronary arteries (INOCA), offering guidance on the diagnostic approach and management. Published simultaneously in European Heart Journal and EuroIntervention by a task force chaired by Vijay Kunadian (Translational and Clinical Research Institute, Newcastle University and Freeman Hospital, Newcastle upon Tyne NHS Foundation Trust, Newcastle upon Tyne, UK) and co-chaired by Alaide Chieffo (IRCCS San Raffaele Scientific Institute, Milan, Italy), it offers the views of an expert panel based on existing evidence from research and best available clinical practice, and notes gaps in knowledge and potential areas for further investigation.
Paragonix Technologies presented new clinical data at the American Transplant Congress (ATC) on the successful use of the Paragonix SherpaPak cardiac transport system for high-risk donor hearts at the Medical University of Vienna (Vienna, Austria), a leading European heart transplant centre.
Ancora Heart has been given US Food and Drug Administration (FDA) approval of its investigational device exemption (IDE) application for the CorCinch-HF pivotal study, which is designed to evaluate the safety and efficacy of the AccuCinch Ventricular Restoration System in patients with heart failure and reduced ejection fraction (HFrEF).
NuVera Medical has announced the initiation of the company’s first-in-human clinical trial to evaluate the performance of its NuVision intracardiac echocardiography (ICE) catheter. The first study participant was successfully treated this month for an atrial septal defect by principal investigator Adrian Ebner (Cardiovascular Department, The Italian Hospital, Asuncion, Paraguay) using remote, live-feed support from the NuVera team who were located in the USA.
US national hospital- and surgeon-level inverse volume–outcome associations were observed for 30-day and one-year mortality after mitral valve surgery for primary mitral regurgitation, according to a study published in JAMA Cardiology. Authors Vinay Badhwar (Department of Cardiovascular and Thoracic Surgery, West Virginia University, Morgantown, USA) and colleagues say the findings may help to define access to experienced centres and surgeons for the management of primary mitral regurgitation.
A subanalysis of the Onyx One study found that cardiac death was significantly higher in patients who received single antiplatelet therapy with a P2Y12 inhibitor following percutaneous coronary intervention (PCI) than those who received single antiplatelet therapy with aspirin. Furthermore, single antiplatelet therapy with a P2Y12 inhibitor was a predictor of the combined endpoint of cardiac death/myocardial infarction/stent thrombosis between one and 12 months.
Left atrial appendage occlusion (LAAO) improves clinical outcomes including ischaemic stroke, major bleeding and all-cause mortality, in atrial fibrillation (AF) patients with a high risk of stroke and major bleeding, compared to the use of novel oral anticoagulants (NOACs). This was the conclusion of a propensity score-matched study presented by Jens Erik Nielsen-Kudsk (Aarhus University, Aarhus, Denmark) at PCR e-Course 2020.
Miracor Medical has announced the award of the CE mark for its latest generation of PiCSO Impulse Catheter and PiCSO Impulse Console, which is indicated for the treatment of anterior STEMI patients. It says the new system features improvements in ease of use.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389