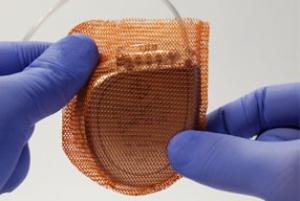
Boston Scientific has today announced it has initiated a controlled launch of the Acurate neo2 aortic valve system in Europe. This next-generation transcatheter aortic valve implantation (TAVI) technology is a new platform designed with a number of features to improve upon the clinical performance of the original Acurate neo platform.
Transcatheter aortic valve implantation (TAVI) device selection should be guided by patient, anatomic, and device-specific factors, a review of the considerations for optimal device choice in aortic valve stenosis has concluded. Key factors to be considered include the likelihood of paravalvular leak (PVL), conduction abnormalities, coronary reaccess, long-term durability, and valve reintervention.
Medtronic has received Breakthrough Device Designation status from the US Food and Drug Administration (FDA) for the Tyrx absorbable antibacterial driveline wrap, which is intended to securely hold a percutaneous driveline in patients receiving a ventricular assist device (VAD).
After surviving a heart attack, the proportion of patients who experience a repeat attack within a year fell between 2008 and 2017, with a greater decline in women than men, according to new research published in the journal Circulation. Despite the improvement, the rate of recurrent heart attacks, hospitalisation for heart failure, and death remains high in heart attack survivors, the study finds.
Abbott has today announced it has received CE mark approval for its fourth-generation MitraClip transcatheter mitral valve repair system. Known as MitraClip G4, the device is now approved for use in Europe and other regions that recognise CE mark as a non-surgical option for the treatment of mitral regurgitation (MR), or a leaky heart valve. The device is already approved for use in the USA.
A study of US Medicare patients undergoing percutaneous coronary intervention (PCI) has found that the use of intravascular ultrasound (IVUS) resulted in a lower long-term mortality, myocardial infarction (MI), and repeat revascularisation. However, the study by Amgad Mentias (University of Iowa Carver College of Medicine, Iowa City, USA) and colleagues, published in JACC: Cardiovascular Interventions, also found that overall use of IVUS guidance cases was low, having been used in just 5.6% of all PCI patients “with a wide variation of its use among different facilities”.
Aspirin-free prasugrel monotherapy following successful everolimus-eluting stent implantation demonstrated feasibility and safety without any stent thrombosis in selected low-risk patients with stable coronary artery disease (CAD). This was the conclusion of the ASET pilot study published in JACC: Cardiovascular Interventions. These findings may help to underpin larger randomised controlled studies to evaluate the aspirin-free strategy compared with traditional dual antiplatelet therapy (DAPT) following percutaneous coronary intervention (PCI), the study’s authors suggest.
VitalConnect has announced the commencement of the TELESTAR-TAVR clinical study—Deployment of Telemedicine for Symptom Tracking And Decrease Readmission Rate in TAVR Patients)—monitoring patients undergoing transcatheter aortic valve replacement (TAVR) using VitalConnect’s VistaSolution LIVE technology. The trial has a primary objective of measuring the impact of remote patient monitoring (RPM) and telehealth on patient satisfaction, clinical outcomes, and readmission rates for patients.
Results of the TIDES-ACS randomised controlled trial, confirming the superior safety and sustained non-inferior efficacy of the Bio-Active Stent technology (Hexacath) at 18 months follow-up versus a drug-eluting stent, have been published.
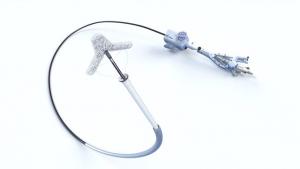
Medtronic has announced US Food and Drug Administration (FDA) approval of an early feasibility study (EFS) of the Intrepid transcatheter tricuspid valve replacement (TTVR) system in patients with severe, symptomatic tricuspid regurgitation. This comes on the heels of a recent Breakthrough Device Designation for the Intrepid TTVR System. The Intrepid TTVR system is an investigational device worldwide.
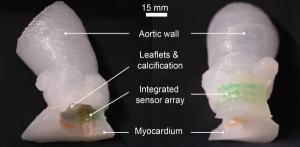
Researchers from the University of Minnesota, with support from Medtronic, have developed a process for multi-material 3D printing of models of the heart’s aortic valve and the surrounding structures that mimic a real patient.
Cardiac Dimensions has announced that Australia’s Therapeutic Goods Administration (TGA) has approved its Carillon Mitral Contour System, a right heart transcatheter mitral valve repair (TMVr) device. The device is designed to treat the primary cause of functional mitral regurgitation (FMR) in patients with mitral regurgitation grades 2+, 3+ and 4+.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389