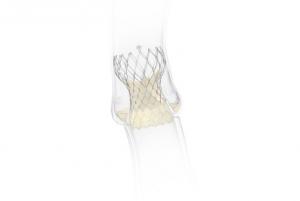
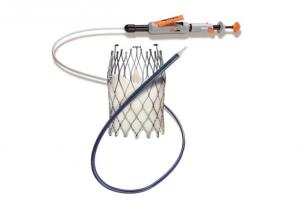
Medtronic has been awarded CE mark for the use of the Evolut transcatheter aortic valve implantation (TAVI) system for patients with severe native aortic stenosis who are at low surgical risk. The Evolut TAVI platform has also received a new indication approval for the treatment of patients with bicuspid aortic valves who are at intermediate, high and extreme risk of surgical mortality.
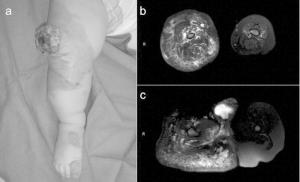
Venous malformations (VMs), like other vascular malformations, are present at birth. They are the most common type of vascular malformation, affecting 1% to 4% of individuals, and clinically appear as a bluish, soft, compressible lesions typically found on the face, limbs, or trunk
Identification and treatment of vascular malformations is a challenging endeavor for physicians, especially given the great concern and anxiety created for patients and their families. The goal of this article is to provide a review of vascular malformations, organized by subtype, including capillary, venous, lymphatic and arteriovenous malformations. Only by developing a clear understanding of the clinical aspects, diagnostic tools, imaging modalities, and options for intervention will appropriate care be provided and results maximized.
The Society for Vascular Surgery (SVS) has released new clinical practice guidelines on the appropriate care and treatment of aneurysms of the visceral arteries.
In a new field safety notice, the UK Medicines and Healthcare products Regulatory Agency (MHRA) states that a warning and clinical summary section will be added to the instructions for use (IFU) of 12 paclitaxel-coated balloons and paclitaxel-eluting stents used in the treatment of peripheral arterial disease (PAD) of the lower limbs.
The first patients have been enrolled in the single-centre DEPPER LIMUSclinical trial, Reflow Medical has announced. The non-randomised pilot study will evaluate the temporary spur stent system for the treatment of lesions in the infrapopliteal arteries using a limus-base drug-coated balloon.
Telemedicine has come into its own during the pandemic. Sameer Mehta details the STEMI treatment strategy of the Latin America telemedicine infarct network (LATIN), and shares lessons learnt.
The British Heart Foundation (BHF) is calling on the Government and the NHS to urgently address the immediate needs of heart and circulatory patients who have had care postponed during the coronavirus pandemic. The charity made its statement after a survey it carried out found thatnearly half (47%) of UK adults with heart and circulatory diseases find it harder to get medical treatment in lockdown.
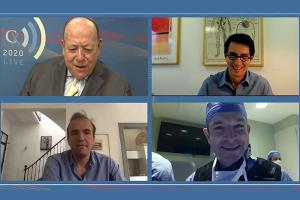
Last week, the CX 2020 LIVE agenda turned to the technically challenging topic of aortic arch interventions. Through presentations, discussion, and polling, the session—chaired by Roger Greenhalgh (London, UK) and moderated by Stéphan Haulon (Paris, France)—aimed to tackle some vital questions, including how to reduce the risk of stroke in thoracic aortic endovascular procedures, what the best options are for the left subclavian branch, and how best to approach aortic arch aneurysms. The session covered atherosclerosis, dissection, transection and ulcers, and their role in guiding treatment was clearly emphasised, with audience polling revealing that 85% would alter reconstruction in the arch according to the underlying pathology. The session also dealt with the wider debate of open versus endovascular surgery and considered the benefits of a multidisciplinary team. In a boost for the endovascular approach in this difficulty anatomy, 63% of audience members disagreed with the statement “Open is best”.
Blue Sail Medical has completed the acquisition of Swiss based company NVT AG. The closure of the deal was publicly announced on the Shenzhen Stock Exchange on the 9 June 2020, and a press release from Blue Sail Medical says it will leverage the capabilities and experience in interventional cardiology of its subsidiary, Biosensors International Group.
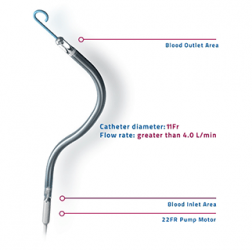
Abiomed has received investigational device exemption (IDE) from the US Food and Drug Administration (FDA) for an early feasibility study with a first-in-human trial of Impella ECP (expandable cardiac power), a 9 French (Fr) heart pump. It will be studied in high-risk percutaneous coronary intervention (PCI) patients. The FDA has also issued an emergency use authorisation (EUA) for Impella RP to include patients suffering from COVID-19 related right heart failure or decompensation, including pulmonary embolism (PE). Impella RP is a temporary heart pump that provides circulatory support for patients who develop right side ventricular failure.
Medtronic has received the CE mark for a one-month dual antiplatelet therapy (DAPT) indication for high bleeding risk patients implanted with the Resolute Onyx drug-eluting stent. A press release reports that for high bleeding risk patients, this new, first-of-its-kind indication allows physicians to recommend a shorter, one-month regimen of DAPT, following a percutaneous coronary intervention (PCI) with Resolute Onyx.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389