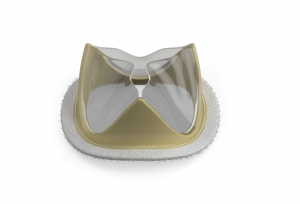
VDYNE has announced today that it has secured a US$21 million Series C financing, proceeds of which will fund first-in-human studies of its tricuspid valve replacement device and delivery system. The financing round was supported by existing investors, including Jean Boulle Medtech, together with significant participation from a large, global medical device company.
A recent systematic review found that “carefully designed and structured” simulation-based training (SBT) in open vascular surgery is effective and can improve technical skills, especially in less experienced trainees. However, authors Jonathan Lawaetz (Rigshospitalet, Copenhagen, Denmark) and colleagues note that the supporting evidence “lacks homogeneity in the reporting standards and types of simulations”. Pass/fail standards that support proficiency-based learning and studies investigating skills transfer “should be the focus on future studies,” they write.
Michael Poon (Lenox Hill Hospital-Northwell Health, New York, USA) discusses the progress made in treatment of cardiovascular disease, and considers how US healthcare practitioners can learn from their overseas colleagues in the use of coronary computed tomography angiogram (CTA), which he describes as the “gold standard” diagnostic technique in many countries.
Okami Medical has revealed the expansion of its LOBO vascular occlusion system product line with US Food and Drug Administration (FDA) 510(k) clearance of the LOBO-5 vascular occluder.
Speaking at the Virtual Aortic Surgery How to Do It (HTDI) Highlights conference (17–18 December), Stéphan Haulon (Hôpital Marie Lannelongue, GHPSJ, Paris, France) discussed total endovascular treatment of the aortic arch in chronic dissections. Detailing global experience using three-vessel inner branch stent grafts for this procedure, Haulon highlighted good technical success and low morbidity and mortality rates. However, he also stressed that the high number of secondary procedures required remains the “Achilles’ heel” of the technique, and that larger experiences and longer follow-up are mandated.
Edwards Lifesciences has announced that the first patient has been treated in the RESTORE clinical trial, which will evaluate the safety and effectiveness of the investigational Harpoon Beating Heart Mitral Valve Repair System in the USA and Canada. The Harpoon system is used to treat severe degenerative mitral valve regurgitation. The procedure took place at the University of Maryland Medical Center, Baltimore, USA.
Edwards Lifesciences has announced that Health Canada has approved the expanded use of the Edwards Sapien 3 and Sapien 3 Ultra transcatheter heart valves for the transfemoral treatment of patients diagnosed with severe symptomatic aortic stenosis who are at low risk for open-heart surgery. The Sapien 3 valves are the first transcatheter aortic valve implantation (TAVI) systems to have this indication in Canada.
Abbott has announced that the US Food and Drug Administration (FDA) has approved updated labelling for the company’s HeartMate 3 heart pump to be used in paediatric patients with advanced refractory left ventricular heart failure. With the updated labelling, physicians now have additional options for treating this underserved population awaiting a heart transplant or for those not eligible to receive a transplant as a result of potential complications or risk related to the procedure.
A subgroup analysis of the ISAR REACT-5—Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5—trial has found that among patients with ST-segment–elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) receiving either prasugrel or ticagrelor, there was no significant difference in combined rates of death, myocardial infarction, or stroke at one year.
New joint clinical practice guidelines on the treatment of patients with valvular heart disease have been issued today by the American College of Cardiology (ACC) and the American Heart Association (AHA). The 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease were published in the AHA’s journal Circulation and in the Journal of the American College of Cardiology.
Foldax has announced that the US Food and Drug Administration (FDA) has granted investigational device exemption IDE approval for the company to initiate a clinical study of its Tria biopolymer mitral surgical heart valve. The company anticipates the first use of its mitral valve in a human will take place in the coming weeks.
The American College of Cardiology (ACC) will collaborate with Amgen and Veradigm on a study to transform care for acute coronary syndrome (ACS) patients at risk for future cardiovascular events. TRANSFORM: Accelerating Lipid Lowering Post ACS (TRANSFORM: ACS) will ensure ACS patients quickly receive cholesterol testing in the hospital and guideline-recommended therapies to reduce LDL cholesterol in the hospital and upon discharge.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389