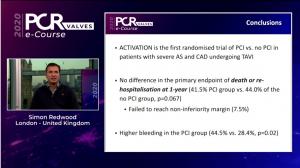
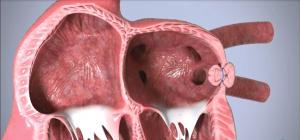
The first randomised trial comparing a course of pre-procedure percutaneous coronary intervention (PCI) versus no PCI in patients undergoing transcatheter aortic valve implantation (TAVI) with significant coronary artery dieseae (CAD) found no difference in the primary endpoints of death or rehospitalisation at one-year follow-up between the two treatment strategies.
Discussion during the first day of the PCR Valves e-course (22–24 November, virtual) centred on the aortic valve and sought to answer key questions over the latest clinical evidence on transcatheter aortic valve implantation (TAVI) in low-risk patients, as well as probing the decision-making process guiding the choice between transcatheter or surgical approaches to aortic stenosis.
A study published in ACS Biomaterials Science & Engineering details the development of a 3D-printed, full-size model of a human heart, that could be used to train surgeons preparing for surgical procedures in the heart. Authored by Eman Mirdamadi and colleagues from the Department of Biomedical Engineering, Carnegie Mellon University (Pittsburgh, USA), the study details the use of Freeform Reversible Embedding of Suspended Hydrogels (FRESH) bioprinting to develop a human heart model from patient-derived magnetic resonance imaging (MRI) datasets.
Corvia has announced completion of randomisation in its REDUCE LAP-HF II global, pivotal trial. The trial is evaluating the Corvia atrial shunt (IASD)—a novel, transcatheter implant—to reduce elevated left atrial pressures (LAP), the primary cause of HF symptoms, in heart failure patients with preserved ejection fraction (HFpEF) or mid-range ejection fraction (HFmrEF). The company also announced authorisation by the US Food and Drug Administration (FDA) to continue evaluation of the Corvia Atrial Shunt under a Continued Access Protocol (CAP) while its pre-market approval (PMA) application is under review. PMA submission is planned for late 2021.
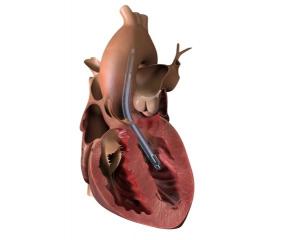
Boston Scientific has announced a global recall of all unused inventory of its Lotus Edge transcatheter aortic valve implantation (TAVI) system due to complexities associated with the product delivery system.
Sinomed has announced the presentation of data from its inter-continental PIONEER III study comparing the safety and efficacy of the Supreme HT (healing-targeted) drug-eluting stent (DES), to the Xience (Abbott) or Promus durable polymer stent (Boston Scientific). One-year results were presented during a late-breaking trial session at the American Heart Association 2020 scientific sessions (AHA 2020, 13–17 November, virtual). The data showed equivalent clinical performance of the Supreme HT to the market-leading DES and will be used to support US Food and Drug Administration (FDA) and Japanese regulatory approvals.
Among patients who stopped taking statins due to side effects, researchers found the statin may not have been the culprit as patients taking a placebo reported the same side effects, according to late-breaking research presented at the American Heart Association’s Scientific Sessions (AHA 2020, 13–17 November, virtual). The study, funded by the British Heart Foundation, was also published in the New England Journal of Medicine (NEJM).
A pooled analysis of interventional studies assessing the feasibility, efficacy and clinical outcomes of transcatheter repair of tricuspid regurgitation (TR) has concluded that a percutaneous repair strategy for severe TR appears to be feasible, effective, and is associated with improved clinical outcomes at mid-term follow-up.
Append Medical, developer of the Appligator implant-free left atrial appendage (LAA) occlusion device, today announced completion of its first pre-clinical chronic procedures. The procedures were completed with the research organisation IMMR and pave the way for the first-in-human trial of the device, Append Medical said in a press release.
Heart valve procedures performed by cardiothoracic surgeons with 10 years of experience or less are associated with a significantly higher risk of mortality compared with those performed by more experienced surgeons. This is the finding of an analysis of the association between cardiothoracic surgeons’ years in practice and operative outcomes on coronary artery bypass grafting (CABG) and valve surgery, published in JAMA Network Open.
Results of the Onyx One Clear study, evaluating the safety and effectiveness of one-month dual antiplatelet therapy followed by single antiplatelet therapy in high bleeding risk (HBR) patients undergoing percutaneous coronary intervention (PCI) using the Resolute Onyx (Medtronic) drug-eluting stent have been published online this week in Circulation: Cardiovascular Interventions. Findings of the study were initially shared virtually at the American College of Cardiology/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual) in March.
Abiomed has announced that the first 1,000 patients have been treated with the Impella 5.5 with SmartAssist heart pump in the first year after the US Food and Drug Administration (FDA) granted approval for the device.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389