A significant decline in percutaneous coronary intervention (PCI) procedures was seen in England early on in 2020 due to the lockdown enforced in the country during the first wave of the COVID-19 pandemic. This is according to a paper published in the journal Circulation: Cardiovascular Interventions looking at the changes in PCI practice throughout England during the pandemic, looking at changes in clinical presentation, characteristics of patients and their clinical outcomes.
The use of ticagrelor in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI) was not associated with a significant difference in the risk of net adverse clinical events (NACE), a study published in the Journal of the American Medical Association (JAMA) has found.
During the prize session at this year’s European Society for Vascular Surgery annual meeting (ESVS Month, 29 September–29 October, virtual), Maaz Syed (University of Edinburgh, Edinburgh, UK) presented early clinical outcomes of a “promising multimodality imaging technique” in acute aortic syndrome—18F-sodium fluoride positron emission tomography (PET).
RaFeVA (Rapid Femoral Vein Assessment), a new protocol, is a rapid and effective tool for the systematic ultrasound evaluation of the veins in the inguinal area and at mid-thigh, conclude Fabrizio Brescia (Unit of Anesthesia and Intensive Care Medicine, Vascular Access Team, Centro di Riferimento Oncologico di Aviano, IRCCS, Aviano, Italy) et al in The Journal of Vascular Access (JVA). Describing this new protocol step-by-step, the study authors say it is designed to evaluate patency and calibre of the common and superficial femoral veins and to help interventionalists choose the best venipuncture site before insertion of a femorally-inserted central catheter (FICC).
Abiomed has announced the treatment of the first two patients using the Impella ECP expandable percutaneous heart pump—a device described by the company as the smallest heart pump in the world. Impella ECP measures 3mm in diameter upon insertion and removal from the body. While in the heart, it expands while supporting the heart’s pumping function, providing peak flows greater than 3.5L/min.
Foldax has announced that the US Food and Drug Administration (FDA) has granted approval to expand the US clinical study of the Tria surgical aortic heart valve. This next stage of enrolment is expected to begin within the next month.
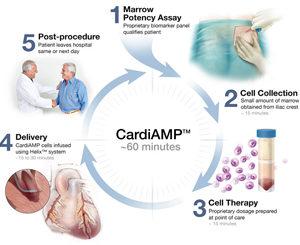
BioCardia has announced the publication of data from the initial open-label roll-in cohort that served as the precursor for the ongoing CardiAMP Heart Failure (HF) phase III clinical trial. Published in the International Journal of Cardiology, the study data shows that CardiAMP is a well-tolerated treatment in heart failure patients and demonstrated improved distance in six-minute exercise testing at six months and a trend toward improvement at one year. This 10-patient cohort was the feasibility test for the currently enrolling CardiAMP HF Phase 3 clinical trial.
A study published in the Journal of the American College of Cardiology (JACC) has identified different types of cardiac structural damage experienced by COVID-19 patients after cardiac injury that can be associated with deadly conditions including heart attack, pulmonary embolism, heart failure, and myocarditis.
Tricupsid annular dilatation (TAD) is an independent predictor of all-cause mortality in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI), a study published in JACC: Cardiovascular Interventions has found.
Rhian E Davies (University of Washington Medical Center, Seattle, USA) discusses a recent paper considering the importance of training in high-risk percutaneous coronary intervention (PCI) procedures, as well as a renewed interest in both the development of this skillset outside of fellowship and maintenance of competency.
Results of the OPTIMIZE IDE study, assessing the Svelte drug-eluting stent (DES) Integrated Delivery System (SLENDER IDS) and Rapid Exchange (DIRECT RX) platforms (Svelte Medical Systems) were presented at TCT Connect 2020 (14 – 18 October, virtual). The study showed that use of the devices resulted in a 1.5% clinically-driven target lesion revascularisation (TLR) at one-year.
Biosensors has announced the award of a CE mark for BioFreedom Ultra drug-coated coronary stent system. BioFreedom Ultra is a novel thin strut (84µm) CoCr polymer and carrier-free drug-coated stent with Biosensors’ proprietary Biolimus A9 drug. BA9 is Biosensors’ proprietary highly lipophilic anti-restenotic drug, developed specifically for use in coronary vascular applications.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389