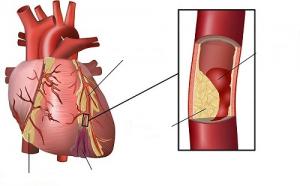
Researchers suggest that in patients with chronic limb-threatening ischaemia (CLTI), two-dimensional (2D) perfusion angiography is a “reliable tool” when used according to standardised methods. Jetty Ipema (St Antonius Hospital, Nieuwegein, The Netherlands) and colleagues write in the European Journal of Vascular and Endovascular Surgery (EJVES) that theirs is the first study to test the reliability of this method in CLTI patients.
Shape Memory Medical recently announced that it has received approval from the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) to market its Impede-FX embolisation plug in Japan. Cosmotec championed the approval process in Japan and is Shape Memory Medical’s distribution partner for its peripheral embolisation products.
SA announced today that the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has approved its Clinical Trial Notification for the Japanese cohort of the ongoing PROMISE IIpivotal trial of the LimFlow percutaneous deep vein arterialisation (pDVA) system.
Medtronic has received US Food and Drug Administration (FDA) approval for its Harmony transcatheter pulmonary valve (TPV), the first minimally invasive therapy created to treat patients with a specific type of congenital heart defect of the right ventricle (RV).
Shockwave Medical has announced that the company has signed an agreement to form a joint venture with Genesis MedTech Group to introduce its intravascular lithotripsy (IVL) technology to the Chinese market. Together, Shockwave and Genesis strive to bring coronary and peripheral IVL products to patients in mainland China, the companies said in a press release.
Three-year results from the COMPARE-ABSORB trial, presented during a late-breaking trial session at the 2021 Cardiovascular Research Technologies meeting (CRT 21 Virtual, 13 February–24 April), indicate that the Absorb (Abbott) bioresorbable scaffold resulted in a similar rate of target lesion failure (TLF) to the Xience (Abbott) metallic stent in patients at high risk of restenosis.
Three-year clinical outcomes from the Recre8 trial, a multicentre study comparing the safety and efficacy of the polymer-free Cre8 (Alvimedica) amphilimus-eluting stent to the Resolute Integrity (Medtronic) permanent-polymer zotarolimus-eluting stent, found that the Cre8 stent was non-inferior to Resolute Integrity at long-term follow-up.
Lab-created heart valves implanted in young lambs were capable of growth within the recipient, a study published in Science Translational Medicine has found. The valves also showed reduced calcification and improved blood flow function compared to animal-derived valves currently used when tested in the same growing lamb model, researchers at the University Of Minnesota Twin Cities, Minneapolis, USA, who conducted the study, reported.
Cardiovascular Systems has announced that the first patients in the USA have been treated with the Wirion embolic protection system. Wirion is a distal embolic protection filter used to capture thrombus and debris that can be associated with all types of peripheral vascular intervention procedures, including atherectomy.
CytoSorbents has announced the filing of an investigational device exemption (IDE) application to conduct the STAR-T clinical study—Safe and Timely Antithrombotic Removal‒Ticagrelor (STAR-T), in the USA to support an initial US Food and Drug Administration (FDA) regulatory approval.
Elixir Medical has today announced commencement of the INFINITY-SWEDEHEART randomised controlled trial (RCT) of the DynamX coronary bioadaptor system, which is described by the company as the first metallic coronary artery implant that adapts to vessel physiology
Clinical outcomes up to one year after transcatheter aortic valve implantation (TAVI) in low-risk patients with symptomatic, severe bicuspid aortic stenosis are consistent with those witnessed during early analysis, which supported the safety of TAVI in this patient group.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389