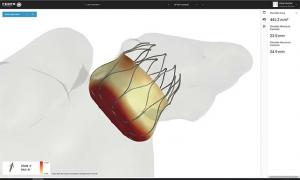
Cook Medical has released de-identifiable patient-level data from a clinical trial of its Zilver PTX peripheral paclitaxel-eluting stent.
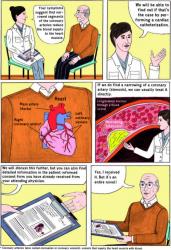
A pilot study, published as a research letter in the Annals of Internal Medicine, has found that patients who received comics or “medical graphic narratives”, as well as standard informed consent protocols (official consent form and a conversation with a physician), had greater comprehension of the coronary angiography procedure that they underwent and less anxiety than those who only received the standard protocols.
PhaseBio Pharmaceuticals, Inc. (Nasdaq: PHAS) has announced that the US FDA has granted breakthrough yherapy designation for PB2452—a novel reversal agent for the antiplatelet drug ticagrelor. Breakthrough therapy designation is designed to expedite the development and review of promising new drugs for serious or life-threatening conditions, when preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints.
TherOx has received US FDA premarket approval for its SuperSaturated Oxygen (SSO2) Therapy. A press release reports that SSO2 Therapy provides interventional cardiologists with the first and only FDA-approved treatment beyond percutaneous coronary intervention (PCI) to significantly reduce muscle damage in myocardial infarction patients.
Avinger has announced that the company received 510(k) clearance from the US Food & Drug Administration (FDA) for its Pantheris SV (small vessel) image-guided atherectomy system.
An analysis on the impact of stroke severity in patients receiving the HeartWare HVAD system (Medtronic) as destination therapy shows that targeted blood pressure management helped reduce serious strokes. The HVAD system is a left ventricular assist device (LVAD) that is designed to increase the amount of blood that circulates through the body in patients with advanced heart failure.
FEops has received CE mark approval for its FEops HEARTguide. According to a press release, FEops HEARTguide is a one-in-its-kind procedure planning environment for structural heart interventions that provides physicians unique insights to evaluate device sizing and positioning preoperatively using novel computational modelling and simulation technology. The current release includes workflows for transcatheter aortic valve implantation (TAVI) and left atrial appendage (LAA) occlusion procedures.
his content is only for readers outside of the USA
In an environment with growing demand for specialist cardiology services, it is important that health tech innovators support physicians to manage demanding workloads and complex procedures while ensuring the patient remains paramount. Within cardiac ultrasound solutions, recent developments are helping to drive improvements not only in terms of patient outcomes but also the user experience. When it comes to ensuring a confident diagnosis, for clinicians, every diagnostic image and patient moment in the care pathway matters.
So, where is the forward momentum heading?
Boston Scientific announced it has received CE mark and initiated a limited market release of the next generation Watchman FLX left atrial appendage closure (LAAC) device in Europe.
The US FDA has approved a new indication for percutaneous edge-to-edge repair (MitraClip, Abbott), allowing its use for the reduction of moderate-to-severe or severe secondary mitral regurgitation in patients who are already receiving optimal medical therapy. When first approved in 2013, the MitraClip was indicated to reduce mitral regurgitation in certain patients with degenerative mitral regurgitation.
Boston Scientific announced it has received CE mark and initiated a limited market release of the next generation Watchman FLX left atrial appendage closure (LAAC) device in Europe.
Daniel J Blackman (Department of Cardiology, Leeds Teaching Hospital NHS Trust, Leeds, UK) and others report in the Journal of the American College of Cardiology (JACC) that in their study, 91% of patients remained free of severe structural valve degeneration between five and 10 years after receiving a transcatheter aortic valve implantation (TAVI) device. They note that they have described the largest cohort of patients with echocardiographic assessment of valve function between five to 10 years.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389