The TASTE and TOTAL studies suggested that routine thombus aspiration in patients undergoing percutaneous coronary intervention (PCI) did not provide mortality benefit and may increase the risk of stroke. However, a new study (CHEETAH) is evaluating whether a next-generation aspiration catheter could lead to improved outcomes. S Jay Mathews (Manatee Memorial Hospital, Bradenton, USA), principal investigator of the study, talks to Cardiovascular News about why thrombus aspiration still has the potential to be an effective tool in PCI.
Preprocedural administration of an oral colchicine load does not reduce the risk of myocardial injury or 30-day major adverse cardiovascular events (MACE) in patients undergoing percutaneous coronary intervention (PCI), according to findings from the COLCHICINE-PCI (Effects of acute colchicine administration prior to percutaneous coronary intervention) clinical trial. However, the study concluded that colchicine could prevent vascular inflammation during an acute injury.
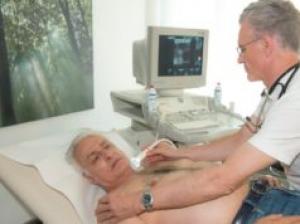
Daiichi Sankyo Europe has announced outcomes from an observational study in mainly caucasian atrial fibrillation (AF) patients being treated with the anti-coagulation drug edoxaban (Lixiana).
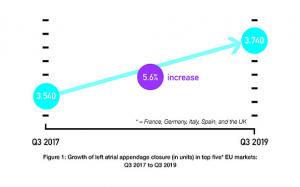
Data from the CAP and CAP2 registries—which contain the longest and largest follow-up of patients—add to previous evidence that left atrial appendage closure (LAAC) with Watchman (Boston Scientific) is a safe and effective alternative to long-term anticoagulation in patients with atrial fibrillation. Furthermore, the results show the lowest rate of haemorrhagic stroke observed in this patient population.
The US Food and Drug Administration has granted Breakthrough Device designation to the digital health and artificial intelligence (AI) company Ekofor an echocardiogram (ECG)-based algorithm to help identify induced Left Ventricular Ejection Fraction (LVEF).
The largest trial to date to compare revascularisation with a conservative strategy in patients with stable ischaemic heart disease has found no additional benefit a median of three years after the procedure.
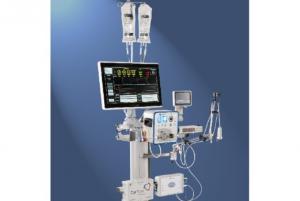
CorFlow Therapeutics has announced that the company has been granted Breakthrough Device designation from the US Food and Drug Administration (FDA) for its Controlled Flow Infusion (CoFI) System. It is indicated for the diagnostic assessment of the coronary microcirculation immediately after percutaneous coronary interventions (PCI) and as a platform for controlled infusion of therapeutic agents into the microcirculation, with or without vessel occlusion.
Penumbra today announced that the EXTRACT-PE trial successfully met the primary endpoints, demonstrating the safety and efficacy of the Indigo Aspiration system for aspiration mechanical thrombectomy in patients with acute pulmonary embolism (PE). The data were presented in a late-breaking clinical trial session today at the 2019 Vascular Interventional Advances conference (VIVA) in Las Vegas, USA (4–7 November).
Two-year data confirm that common femoral artery (CFA) disease can be treated endovascularly with the Supera stent (Abbott). Results of the VMI-CFA study were presented today at the 2019 Vascular and InterVentional Advances meeting in Las Vegas, USA (4–7 November).
William W Pinsky and Mandeep R Mehra explain to Cardiovascular Newshow international medical graduates contribute to both their adopted country and their country of origin, providing benefits for all.
Cardionovum’s Restore paclitaxel-coated balloon for coronary applications has received market approval for use in China. It can be used in treating two indications: in-stent restenosis and small vessel disease. A joint press release from Cardionovum and China Grand Pharmaceutical and Healthcare Holdings states that the Restore device features the company’s SafePax next-generation, stable, homogeneous, paclitaxel coating. It says that SafePax is an amorphous, noncrystalline matrix that is not affected by mechanical stress due to elastic, lipophilic, polymeric excipients.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389