Who should have a Cardiac CT?
Before considering undergoing a cardiac CT, the most important step for the patient is to consult with their physician. This is because some cardiac CT uses are more appropriate than others, and the scan carries some risks from X-ray exposure and contrast dye administration.
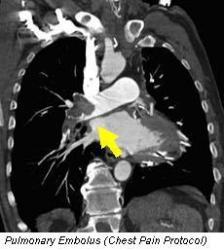
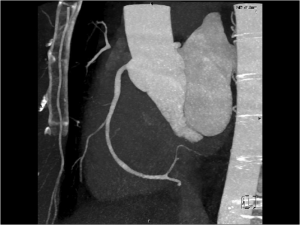
In line with our commitment to provide world-class facilities and deliver excellence in clinical care, San Radiology recently installed the cardiac-capable Siemens FORCE CT Scanner (384-slice, Dual Source). In March 2015, in a world-first, San Radiology achieved the lowest dose of radiation ever reported for Cardiac CT imaging, according to the supplier Siemens Healthcare. Our average dose for Cardiac CT is significantly lower than the safety reference guidelines used for CT in Australia (in many instances up to 98% lower).
Medtronic recently announced the global launch of the Telescope guide extension catheter, a newly designed catheter used to provide additional backup support and access to distal lesions. Guide extension catheters help deliver coronary stents, balloons and other interventional devices during angioplasty procedures that help to restore blood flow through the coronary and peripheral arteries.
Royal Philips recently announced the launch of the new IntraSight interventional applications platform. The secure, application-based platform offers a comprehensive suite of clinically proven iFR, FFR, IVUS and co-registration modalities to simplify complex interventions and speed routine procedures.1
Ortho Clinical Diagnostics has received the CE mark for its next-generation Vitros high sensitivity troponin I assay. A press release reports that this new assay is the latest addition to the company’s robust cardiology menu and is a critical tool for clinicians who are seeking improved strategies to more rapidly and accurately identify patients suffering from a heart attack. It adds that the assay also aids in identifying low-risk patients who may be safely discharged to help reduce the cost of care and alleviate the burden on hospital resources.
Boston Scientific has received US FDA approval for its Lotus Edge transcatheter aortic valve implantation (TAVI) system. It is approved for patients with severe aortic stenosis who are considered at high risk for surgical valve replacement via open heart surgery. Prior to the approval, the only TAVI devices on the US market were Medtronic’s CoreValve and Edwards Lifesciences’ Sapien 3/Sapien XT.
Patients with ST segment elevation myocardial infarction (STEMI) who undergo percutaneous coronary intervention (PCI) should be triaged for intensive care (ICU) treatment based on risk factors that include reperfusion delay to avoid overuse of ICU facilities. A review of patterns of ICU use found that although >80% of stable patients with STEMI are treated in the ICU after primary PCI, the risk for developing a complication requiring ICU care is 16%.
Nearly two thirds (64.3%) of cath lab staff believe that the financial/administrative burdens of meeting percutaneous coronary intervention (PCI) public reporting requirements are not outweighed by the potential benefits in terms of improving quality of care. Furthermore, the median institutional costs to meet public reporting requirements are US$100,000 to US$200,000 and interventional cardiologists spend a median time of five to 10 hours per week on meeting these requirements.
Using EndoAnchors to fix and seal endovascular aortic grafts is safe and effective for short-neck abdominal aortic aneurysm (AAA) patients, two-year data from the ANCHOR global registry have shown. Frank Arko (Charlotte, USA) presented the findings at the 2019 Charing Cross Symposium (CX; 15–18 April, London, UK) in a Podium 1st session, confirming a high success rate for the procedure. “The goal of contemporary AAA therapy is the long-term durability of the repair. Surgical repair does this very well. Can we simulate and get those same results with endovascular repair, with the addition of EndoAnchors? I tend to think so, and I think that today would also suggest that as well.”
NuCryo Vascular, the manufacturer and marketer of the PolarCath Balloon Dilatation System, announced today that the company is raising a Series B round of financing and is launching its Extended PolarCath Balloon Dilatation Catheter.
CX Executive Board member Una Adderly (Wakefield, UK), director of the National Wound Care Strategy Programme for the National Health Service (NHS) England, spoke recently at the 2019 Charing Cross Symposium (CX; 15–18 April, London, UK) on the challenge of ankle-brachial pressure index (ABPI) for managing vascular disease. Adderly argued for the benefits of introducing ABPI assessment, claiming the data supports that a significant majority of amputations due to chronic vascular disease could be prevented with its use.
Steven Rogers (Manchester, UK) presented the case for using contrast-enhanced tomographic 3D ultrasound imaging for peripheral arterial disease, telling the audience at Charing Cross Symposium (CX; 15–18 April, London, UK): “Our surgeons certainly prefer the 3D vein mapping to a 2D vein map”.

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389